MOF 衍生的掺杂 N 的 CoNi@C 作为双功能催化剂用于高效水分离†。
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
开发用于氧进化反应(OER)和氢进化反应(HER)的高效、稳定的双功能非贵金属催化剂对于电化学水分离至关重要。本研究以 ZIF-67 为原料,通过高温煅烧合成了 CoNi@C 核壳结构催化剂。亲水性碳壳抑制了 Co 和 Ni 的氧化,提高了水分离反应的活性和稳定性。CoNi 是反应的活性位点,而掺杂的氮则进一步促进了电催化反应。合成的 Ni1Co10/C 催化剂具有很高的双功能性能,在 100 mA cm-2 时,OER(380 mV)和 HER(357 mV)的过电位都很低,而且双层电容很高(54 mF cm-2)。此外,Ni1Co10/C 催化剂还是双电极系统中有效的水分离阴极,电池电压稳定在 2.05 V,并在 24 小时内保持 100 mA cm-2 的恒定催化电流。DFT 计算表明,OER 和 HER 的活性位点是 Co.本文章由计算机程序翻译,如有差异,请以英文原文为准。
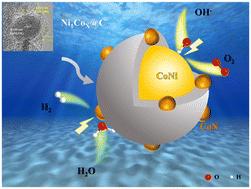
MOF-derived N-doped CoNi@C as bifunctional catalysts for efficient water splitting†
The development of efficient and stable bifunctional non-noble metal catalysts for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) is essential for electrochemical water splitting. In this study, CoNi@C core–shell structure catalysts derived from ZIF-67 were synthesized through high-temperature calcination. The hydrophilic carbon shell inhibits the oxidation of Co and Ni and enhances the activity and stability of the water splitting reaction. CoNi serves as the active site of the reaction, while the doped nitrogen further promotes the electrocatalytic reaction. The synthesized Ni1Co10/C catalyst exhibits high bifunctional performance, with low overpotentials at 100 mA cm−2 for the OER (380 mV) and HER (357 mV), and a high double-layer capacitance (54 mF cm−2). Moreover, the Ni1Co10/C catalyst serves as an effective cathode for water splitting in a two-electrode system, demonstrating a stable cell voltage of 2.05 V and maintaining a constant catalytic current of 100 mA cm−2 over 24 hours. DFT calculation showed that the active site of the OER and HER was Co.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


