可调节、原位生成的镍氢烯烃异构化催化剂†。
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们利用耐空气试剂开发了一种模块化、操作实用的 Ni(0)/silane 烯烃异构化系统。该方法的一个吸引人的特点是能够快速筛选各种 N-杂环碳烯 (NHC) 配体,通过对配体骨架进行辅助修饰来优化配体,从而提高反应活性。该系统易于扩展(1 克烯),并能以高 E 选择性容忍各种官能团,包括芳基溴化物、杂环、叔胺和 α,β-不饱和酰胺。初步的机理实验支持 Ni-H 插入/消除途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
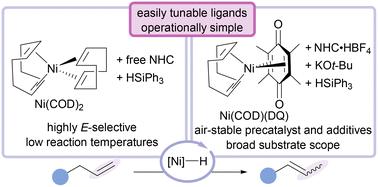
Tuneable, in situ-generated nickel-hydride alkene isomerisation catalyst†
A modular, operationally practical Ni(0)/silane alkene isomerisation system has been developed with air-tolerant reagents. An attractive feature of this method is the ability to rapidly screen a variety of N-heterocyclic carbene (NHC) ligands, enabling optimisation through ancillary ligand backbone modification for enhanced reactivity. This system is readily scalable (>1 g alkene) and tolerates a diverse array of functional groups in high E-selectivity, including aryl bromides, heterocycles, tertiary amines, and α,β-unsaturated amides. Preliminary mechanistic experiments support a Ni–H insertion/elimination pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


