照亮甲烷干转化--探索可见光对 Co/xCeO2-Al2O3† 上碳形成的影响
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在热 DRM 中引入光可能是提高催化剂稳定性的有效策略,但光在稳定性机理中的作用尚不十分清楚。本研究通过合成几种钴浸渍(10 wt% Co)的 xCeO2-Al2O3 载体(xCe-Al,x = 0、5、10 和 20 mol%),系统地调节了载体的碱性和氧释放能力,以研究光对碳形成以及 CHx 氧化和脱氢速率(x = 0-3)的影响。支持物的还原性和二氧化碳吸收量随 Ce 含量的增加而增加,这是因为表面还原时产生了氧空位。Co/Al 的活性最高(650 °C 时 CO2 转化率为 29%,CH4 转化率为 18%),活性和选择性(H2/CO)随 Ce 浓度的增加而降低,这是由于铈具有 RWGS 倾向和/或加入 Ce 后 Co 的尺寸增加(还原后,Co/Al 的尺寸从 13 纳米增加到 Co/20Ce-Al 的 36 纳米)。Co/Al 因碳积累(4.6 wt%,7 小时稳定性测试,650 °C)而失活最严重,因为支撑物没有为 CHx 氧化(x = 0-3)提供 O。由于铈具有从氧空位释放氧气的能力(Co/5Ce-Al 为 0.78 wt%),因此引入 Ce 可提高碳含量和热条件下的稳定性。可见光(2.0 W cm-2)提高了 Co/xCe-Al 的 CH4 转化率,但 CHx 脱氢速率的提高加速了所有催化剂的碳沉积,损害了其稳定性。原位 DRIFTS 没有发现 CHxO 中间体,这表明相对于 CHx 脱氢,CHx 氧化率较低。这些发现突出表明,要促进 CHx 氧化,必须有足够的支撑氧释放,从而实现光促进稳定性的提高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
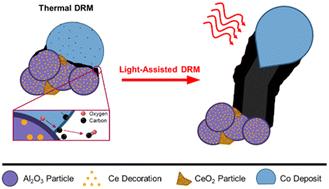
Shining a light on methane dry reforming – exploring the impact of visible light on carbon formation over Co/xCeO2–Al2O3†
Introducing light to thermal DRM may be an effective strategy to improve catalyst stability, but light's role in the stability mechanism is not well understood. This study systematically moderated the support's basicity and oxygen release capacity by synthesising several cobalt-impregnated (10 wt% Co) xCeO2–Al2O3 supports (xCe–Al, x = 0, 5, 10 and 20 mol%) to investigate light's impact on carbon formation and the CHx oxidation and dehydrogenation rates (x = 0–3). The support's reducibility and CO2 uptake increased with Ce content, arising from oxygen vacancies created upon surface reduction. Co/Al was the most active (29% CO2 and 18% CH4 conversion at 650 °C), and the activity and selectivity (H2/CO) decreased with Ce concentration due to ceria's propensity for RWGS and/or the Co size increase upon Ce incorporation (after reduction, from 13 nm for Co/Al to 36 nm for Co/20Ce–Al). Co/Al deactivated the most by carbon accumulation (4.6 wt%, 7 h stability test, 650 °C), as the support provided no O for CHx oxidation (x = 0–3). Introducing Ce improved the carbon content and stability under thermal conditions due to ceria's oxygen release capacity from oxygen vacancies (0.78 wt% for Co/5Ce–Al). Visible light (2.0 W cm−2) improved CH4 conversion of Co/xCe–Al, but the increased CHx dehydrogenation rate accelerated carbon deposition for all catalysts, harming the stability. In situ DRIFTS identified no CHxO intermediate, suggesting poor CHx oxidation rates relative to CHx dehydrogenation. These findings highlight that sufficient support oxygen release is necessary to facilitate CHx oxidation, achieving light-facilitated stability improvements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


