高多孔氮化硼作为二氧化碳与环氧化物环化反应的无金属异相催化剂†。
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
氮化硼作为一种新兴的异相催化剂备受关注。本研究采用热分解法,以不同摩尔比的硼酸(B)和尿素(U)合成了多孔氮化硼催化剂,并将其作为无金属催化剂用于 CO2 与环氧化物的环加成反应。合成的氮化硼样品具有湍流结构,其多孔性能,如表面积、孔径分布和孔体积,在很大程度上取决于 B :U 前驱体的摩尔比。以 B :U = 1 :在用硼酸和尿素制备的样品中,以 B : U = 1 : 5 的摩尔比合成的样品孔体积最大,在四丁基溴化铵(TBAB)存在下,其 CO2 与环氧化物、环氧氯丙烷和氧化苯乙烯的环加成活性最高。相应的碳酸盐产量与催化剂的孔性能之间存在良好的相关性。在合成过程中加入三聚氰胺(M)可大大发展多孔结构,表面积超过 1000 m2 g-1。以 B :M :U = 1 :1 :5 的样品具有 1380 m2 g-1 的大表面积和 1.8 mL g-1 的高孔隙率,在环氧氯丙烷反应中的收率高达 96%。该催化剂可重复使用至少三次,且活性不会明显降低。多孔氮化硼催化剂的羟基作为弱勃氏酸位点使环氧化物的氧原子极化,TBAB 的溴离子作为路易斯碱位点通过亲核攻击激活环氧化物的碳原子,从而提出了一种协同反应机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
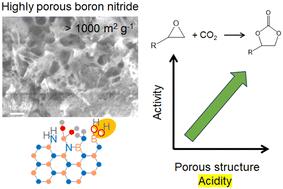
Highly porous boron nitride as a metal-free heterogeneous catalyst for cycloaddition of CO2 to epoxides†
Boron nitride has received much attention as an emerging heterogeneous catalyst. Porous boron nitride catalysts were synthesized using boric acid (B) and urea (U) at different molar ratios via a pyrolysis method and applied for cycloaddition of CO2 to epoxides as metal-free catalysts. The synthesized boron nitride samples had a turbostratic structure, and their porous properties, such as surface area, pore size distribution, and pore volume, largely depended on the molar ratio of B : U precursors. The sample synthesized at a molar ratio of B : U = 1 : 5 with the highest pore volumes among the samples prepared from boric acid and urea exhibited the highest activity for cycloaddition of CO2 to epoxides, epichlorohydrin and styrene oxide in the presence of tetrabutylammonium bromide (TBAB). There was a good correlation between the corresponding carbonate yield and the pore properties of the catalyst. The addition of melamine (M) during the synthesis greatly developed the porous structure, exceeding 1000 m2 g−1 surface area. The sample synthesized at a molar ratio of B : M : U = 1 : 1 : 5 having a large surface area of 1380 m2 g−1 with a high pore volume of 1.8 mL g−1 afforded a remarkable yield of 96% for the reaction of epichlorohydrin. The catalyst could be reused at least three times without a significant loss of activity. A cooperative reaction mechanism was proposed in which the hydroxyl groups of porous boron nitride catalysts as weak Brønsted acid sites polarize the oxygen atom of the epoxide, and the bromide ions of TBAB as Lewis base sites activate the carbon atom of the epoxide by the nucleophilic attack.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


