黄体成纤维细胞通过 MAPK 介导的方式对 IL1β 产生前列腺素。
IF 3.8
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
摘要
黄体是一种临时性的内分泌腺,对怀孕至关重要,因为它能分泌孕激素,维持子宫着床所需的最佳子宫条件。如果没有孕囊,黄体就会失去功能,并迅速发生组织重塑,退化为纤维化的黄体。早期黄体退化的特点是细胞因子释放增加。由于成纤维细胞在牛黄体中的作用仍有待阐明,本研究旨在阐明牛黄体成纤维细胞对炎症细胞因子肿瘤坏死因子α(TNFα)和白细胞介素1β(IL1β)的反应。这两种细胞因子通过ERK1/2、p38 MAPK和JNK的磷酸化诱导黄体成纤维细胞中的典型丝裂原活化蛋白激酶(MAPK)信号转导。IL1β 提高了细胞膜磷脂酶 A2(cPLA2)的表达和磷酸化,这种酶能动员花生四烯酸用于合成类前列腺素。IL1β 还能提高前列腺素内过氧化物合成酶 2(PTGS2)的表达,这是合成前列腺素所需的另一种酶。IL1β 使培养基中的 PGF2α 和 PGE2 水平增加了 20 倍以上。用小分子抑制剂抑制 MAPK 可减弱 IL1β 的刺激作用。IL1β 还能诱导类固醇生成细胞产生前列腺素,但 cPLA2 没有升高。因此,IL1β的作用因卵巢细胞类型而异。综上所述,我们发现黄体成纤维细胞是黄体退化过程中潜在的炎症介质。本文章由计算机程序翻译,如有差异,请以英文原文为准。
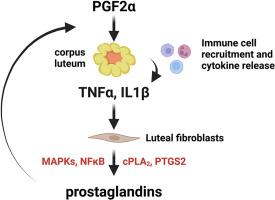
Luteal fibroblasts produce prostaglandins in response to IL1β in a MAPK-mediated manner
The corpus luteum is a temporary endocrine gland that is crucial for pregnancy, as it produces the progesterone needed to maintain optimal uterine conditions for implantation. In the absence of a conceptus, the corpus luteum becomes non-functional and undergoes rapid tissue remodeling to regress into a fibrotic corpus albicans. Early luteal regression is characterized by increased cytokine release. Because the role of fibroblasts in the bovine corpus luteum remains to be elucidated, the aim of this study was to elucidate the response of bovine luteal fibroblasts to inflammatory cytokines, tumor necrosis factor α (TNFα), and interleukin 1β (IL1β). Both cytokines induced canonical mitogen activated protein kinase (MAPK) signaling in luteal fibroblasts by phosphorylation of ERK1/2, p38 MAPK, and JNK. IL1β elevated expression and phosphorylation of cytosolic phospholipase A2 (cPLA2), an enzyme that mobilizes arachidonic acid for prostanoid synthesis. IL1β also elevated expression of prostaglandin-endoperoxide synthase 2 (PTGS2), another enzyme needed to synthesize prostanoids. IL1β increased PGF2α and PGE2 levels in the culture medium over 20-fold. Inhibition of MAPKs with small-molecule inhibitors abrogated the stimulatory effects of IL1β. IL1β also induced prostaglandin production in steroidogenic cells; however, there was no elevation in cPLA2. Therefore, actions of IL1β differ based on ovarian cell type. All together, we have identified luteal fibroblasts as potential inflammatory mediators during luteal regression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


