COL8A1 过表达通过激活局灶粘附激酶信号级联促进胶质瘤细胞生长
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
我们探讨了胶原 VIII 型 alpha-1 链(COL8A1)在胶质瘤中的表达和生物学作用。生物信息学分析表明,胶质瘤组织中 COL8A1 的过表达与患者的不良临床预后相关。在局部胶质瘤组织和各种胶质瘤细胞中也检测到了 COL8A1 的过表达。在原代和永生化胶质瘤细胞中,COL8A1 shRNA 或基因敲除(KO)可降低细胞活力、增殖和移动性,破坏细胞周期并促使细胞凋亡。而 COL8A1 的过表达会增强胶质瘤细胞的恶性行为。原代胶质瘤细胞中的 COL8A1 shRNA 或 KO 降低了 FAK 及下游靶点 Akt 和 Erk1/2 的磷酸化。相反,提高 COL8A1 的表达则会增加它们的磷酸化。体内实验证实,在 COL8A1 KO 后,小鼠脑内来源于患者的胶质瘤异种移植物的生长受到抑制。在 COL8A1 KO 的颅内胶质瘤异种移植物中观察到增殖受阻、FAK、Akt 和 Erk1/2 磷酸化水平降低以及细胞凋亡增加。因此,COL8A1的过表达会促进胶质瘤细胞的生长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
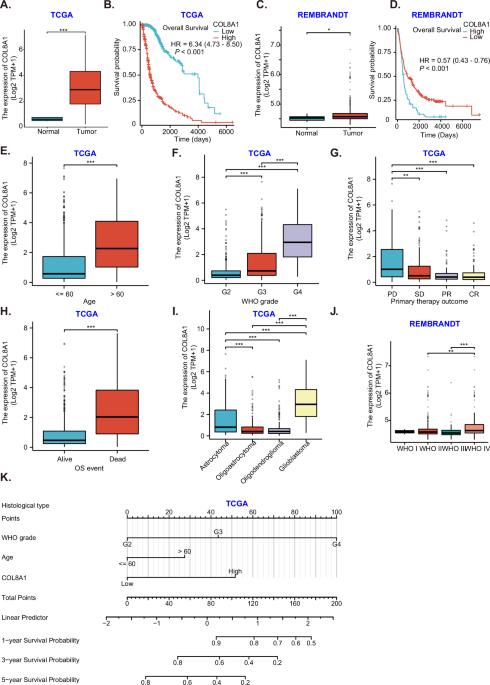
COL8A1 overexpression promotes glioma cell growth by activating focal adhesion kinase signaling cascade
We explored expression and biological roles of collagen type VIII alpha-1 chain (COL8A1) in glioma. Bioinformatics analyses unveiled COL8A1 overexpression within glioma tissues correlates with adverse clinical outcomes of patients. COL8A1 overexpression was also detected in local glioma tissues and various glioma cells. In primary and immortalized glioma cells, COL8A1 shRNA or knockout (KO) reduced cell viability, proliferation and mobility, disrupted cell cycle, and prompted apoptosis. While COL8A1 overexpression augmented the malignant behaviors in glioma cells. COL8A1 shRNA or KO in primary glioma cells decreased phosphorylation of FAK and downstream targets Akt and Erk1/2. Conversely, elevating COL8A1 expression increased their phosphorylations. In vivo experiments confirmed growth inhibition of patient-derived glioma xenografts within the mouse brain following COL8A1 KO. Hindered proliferation, lowered phosphorylation levels of FAK, Akt, and Erk1/2, as well as increased apoptosis were observed within the COL8A1 KO intracranial glioma xenografts. Thus, COL8A1 overexpression promotes glioma cell growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Precision Oncology
ONCOLOGY-
CiteScore
9.90
自引率
1.30%
发文量
87
审稿时长
18 weeks
期刊介绍:
Online-only and open access, npj Precision Oncology is an international, peer-reviewed journal dedicated to showcasing cutting-edge scientific research in all facets of precision oncology, spanning from fundamental science to translational applications and clinical medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


