酰基硅烷的布鲁克氧化反应:α-酮酰胺和α-硫代酰胺的一般获取途径
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们首次成功开发了一种由氨基甲酰基阴离子引发的新型酰基硅烷化学选择性布鲁克氧化反应。这种方法能够在无过渡金属和无强氧化剂的条件下合成各种 α-酮酰胺和 α-硫代酰胺,并具有高产率和高化学选择性。此外,它还对多种官能团具有耐受性。该工艺成功应用于从酰基硅烷合成奥曲肽受体拮抗剂,凸显了其在开发新型治疗药物和进一步探索合成化学方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
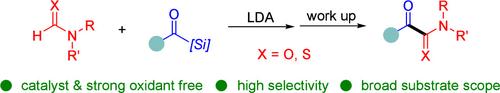
Brook-Oxidation Reaction of Acylsilanes: General Access to α-Ketoamides and α-Ketothioamides
A novel chemoselective Brook-oxidation reaction of acylsilanes initiated by the carbamoyl anion has been successfully developed for the first time. This method enables the synthesis of diverse α-ketoamides and α-ketothioamides under transition metal-free and strong oxidant-free conditions with high yields and high chemoselectivity. It also demonstrates tolerance toward a wide range of functional groups. The synthetic utility of this process is underscored by its successful application in the synthesis of an orexin receptor antagonist from acylsilane, highlighting its potential for the development of novel therapeutic agents and further exploration in synthetic chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


