索泰特受体:肺动脉高压领域的新药。
IF 3.5
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
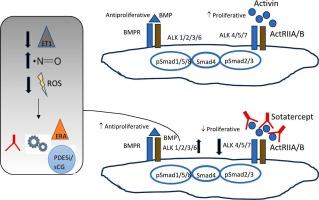
索泰特受体(Sotatercept,品牌名 WINREVAIR,由默克公司开发)是一种激活素受体 IIA 型-Fc(ActRIIA-Fc),通过封存游离激活素发挥作用。Sotatercept 可恢复肺动脉盘旋中激活素增殖途径与骨形态发生蛋白(BMP)抗增殖途径之间的平衡。Sotatercept 最近获得了美国和欧洲的批准,用于治疗肺动脉高压(PAH)1 组成人患者,在 PAH 背景治疗的基础上增加运动能力,改善 WHO 功能分级,降低临床恶化事件的风险。然而,目前仍有多项研究在调查该药物的潜在不良反应,尤其是在血液学方面。我们综述了通过索特拉克(sotatercept)靶向作用途径治疗 PAH 的临床和临床前证据。我们还讨论了索泰特受体在 PAH 第 1 组以外的肺动脉高压中应用的其他可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sotatercept: New drug on the horizon of pulmonary hypertension
Sotatercept (brand name WINREVAIR, developed by Merck) is an activin receptor type IIA-Fc (ActRIIA-Fc), working by sequestering free activins. Sotatercept restores the balance between the activin proliferative pathway and the bone morphogenic protein (BMP) antiproliferative pathway in the pulmonary arterial cirulation. Sotatercept recently received approval in the USA and in Europe for the treatment of adults with pulmonary arterial hypertension (PAH) Group 1, on top of background PAH therapy to increase exercise capacity, improve WHO functional class and reduce the risk of clinical worsening events. Nevertheless, several studies are ongoing to investigate the potential adverse reactions of the drug especially at the haematological level. We provide an overview of the clinical and preclinical evidence of the targeting the activing pathway through sotatercept on the treatment of PAH. We also discuss what other possibilities there are for the application of sotatercept in the setting of pulmonary hypertension other than PAH Group 1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Vascular pharmacology
医学-药学
CiteScore
6.60
自引率
2.50%
发文量
153
审稿时长
31 days
期刊介绍:
Vascular Pharmacology publishes papers, which contains results of all aspects of biology and pharmacology of the vascular system.
Papers are encouraged in basic, translational and clinical aspects of Vascular Biology and Pharmacology, utilizing approaches ranging from molecular biology to integrative physiology. All papers are in English.
The Journal publishes review articles which include vascular aspects of thrombosis, inflammation, cell signalling, atherosclerosis, and lipid metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


