Verteporfin 联合 ROCK 抑制剂可促进大鼠角膜内皮细胞功能障碍的恢复。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
角膜内皮细胞(CECs)功能障碍经常会导致角膜内皮失代偿,造成角膜混浊、水肿,是角膜失明的主要原因。药物干预为角膜移植手术提供了一种创伤较小的替代方法。然而,内皮细胞向间质转化(EndMT)限制了角膜内皮细胞的功能。大量研究表明,Rho-激酶(ROCK)抑制剂是治疗角膜上皮细胞功能障碍的辅助疗法,但它不能完全逆转炎症性环境损害引起的病理性内皮细胞转化。Verteporfin(VP)是Yes相关蛋白(YAP)的抑制剂,对细胞纤维化和间质转化有显著的抑制作用。在此,我们探讨了 VP 在 ROCK 抑制剂治疗角膜内皮功能障碍过程中减轻内膜间质转化的作用。我们通过手术构建了一个 CECs 损伤大鼠模型,并使用 RNA-Seq 测序、免疫荧光、光学相干断层扫描和 qPCR 研究了 VP 和 ROCK 抑制剂对 EndMT、细胞增殖和角膜水肿的影响。结果表明,YAP 在人类胎儿 CECs 中的表达高于成人,并且随着年龄的增长而降低。此外,人类 CECs 中 YAP 的表达与 AQP1 和 ATP1A1 等功能基因呈负相关。VP 能有效逆转角膜内膜移植并加速角膜水化退行。但是,它抑制了 CECs 的增殖。我们还证实,VP 与 Y-27632(ROCK 抑制剂)的最佳配比为 1:1,通过下调 TGF-β 信号转导下游的转录因子,促进 CECs 增殖并逆转 EndMT,从而增加 CECs 功能蛋白和细胞间粘附蛋白。这些综合效应促进了角膜内皮损伤的修复,提供了一种新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
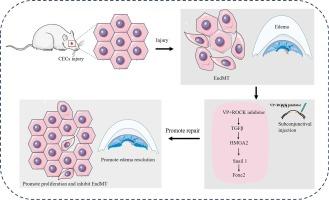
Verteporfin combined with ROCK inhibitor promotes the restoration of corneal endothelial cell dysfunction in rats
Corneal endothelial cells (CECs) dysfunction frequently results in a hazy, edematous cornea due to corneal endothelial decompensation and is a major cause of corneal blindness. Drug interventions provide a less invasive alternative to corneal transplantation surgery. However, endothelial-to-mesenchymal transition (EndMT) limits CECs function. Rho-kinase (ROCK) inhibitors, shown in numerous studies to be an adjunctive therapy for CECs dysfunction, cannot completely reverse pathological EndMT caused by inflammatory environmental damage. Verteporfin (VP) is an inhibitor of Yes-associated protein (YAP) and has significant inhibitory effects on cell fibrosis and mesenchymal transition. Here, we explored VP’s utility in mitigating EndMT during ROCK inhibitors treatment of corneal endothelial dysfunction. We surgically constructed a rat model of CECs injury and studied VP and ROCK inhibitors’ effects on EndMT, cell proliferation, and corneal edema using RNA-Seq sequencing, immunofluorescence, optical coherence tomography, and qPCR. The results indicated that YAP expression in human fetal CECs was higher than in adults and decreased with age in rats. Moreover, YAP expression in human CECs was negatively correlated with functional genes, such as AQP1 and ATP1A1. VP effectively reversed EndMT and accelerated corneal hydration regression. However, it inhibited CECs proliferation. We also confirmed that the optimal ratio of VP combined with Y-27632 (ROCK inhibitor) was 1:1, promoting CECs proliferation and reversing EndMT by down-regulating transcription factors downstream of TGF-β signaling, thereby increasing CECs functional and intercellular adhesion proteins. These combined effects promote corneal endothelial damage repair, providing a new treatment strategy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


