马福明霉素 A 和 D 的全合成
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报告了采用固相方法合成抗菌环肽 marformycin A (1) 和 marformycin D (2)的情况。此外,还介绍了以顺式羟基脯氨酸为起点,溶液相合成 2 中的γ-羟基哌嗪酸亚基的可扩展方法。对 1 及其 Leu-epi 同源物的结构分析表明,构象上的差异可能是它们抗菌活性不同的原因。所述方法有助于进一步发展这类去肽天然产物的构象-活性关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
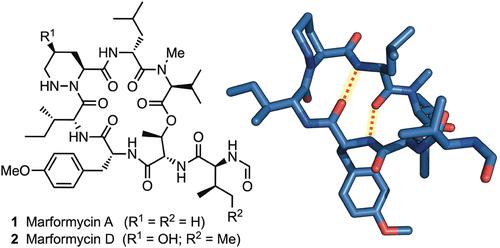
Total Synthesis of Marformycins A and D
We report the synthesis of the antimicrobial cyclodepsipeptides marformycin A (1) and marformycin D (2) using a solid-phase approach. A scalable solution-phase synthesis of the γ-hydroxypiperazic acid subunit in 2, starting from cis-hydroxyproline, is also described. Structural analysis of 1 and its Leu-epi congener demonstrates conformational differences that may underlie their divergent antimicrobial activities. The described approach enables further development of conformation–activity relationships within this class of depsipeptide natural products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


