受挫路易斯对活化甲基三氧铼的烯烃 Metathesis 作用
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
以 Al2O3 或 SiO2-Al2O3 为载体的甲基三氧硼铼 (MTO) 是一种高效的异相烯烃偏析催化剂,可在室温下工作,并能耐受各种官能团。表面研究发现,MTO 与高度路易斯酸性的铝中心相互作用,其甲基可能被 C-H 活化,形成铼-亚甲基物种。催化剂静止态和活性物种的确切结构还存在科学争议。在此,我们报告了 2,6-丁烷和三(五氟苯基)硼烷 (B(C6F5)3)(一种溶液中的受挫路易斯对 (FLP))对 MTO 的活化。MTO/FLP 催化剂在温和条件下对降冰片烯的开环偏聚和内部烯烃的交叉偏聚具有活性。ESI-MS 和 NMR 研究发现,MTO 在 FLP 的存在下会发生去质子化反应,生成一种铼-亚甲基。虽然这种最初被活化的亚甲基没有被检测到,但在 ESI-MS 中喷洒含有结构受限烯烃的反应混合物,可以检测到循环中的铼-亚烷基物种。时程测量结果表明,催化活性不高可归因于催化剂的快速失活步骤。其中一个可能的失活途径是具有元合成活性的亚甲基的第二个去质子化步骤,生成铼-亚甲基。动力学实验表明,它可以在溶液中通过质子化作用重新激活,用于烯烃的偏析反应。此外,我们还假设了几种导致催化剂永久失活的不可逆失活途径。我们认为,MTO/FLP 系统是异相 MTO 催化剂的均相模型系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
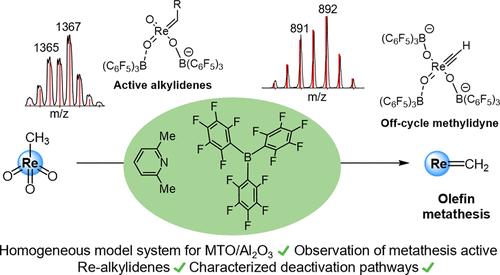
Activation of Methyltrioxorhenium for Olefin Metathesis by a Frustrated Lewis Pair
Methyltrioxorhenium (MTO) supported on Al2O3 or SiO2–Al2O3 is an efficient heterogeneous alkene metathesis catalyst that works at room temperature and tolerates various functional groups. Surface studies found that MTO interacts with highly Lewis-acidic aluminum centers and that its methyl group is probably C–H activated resulting in rhenium-methylidene species. The exact structure of the catalyst resting state and the active species is subject to scientific debate. Here, we report on the activation of MTO by 2,6-lutidine and tris(pentafluorophenyl)borane (B(C6F5)3), a frustrated Lewis pair (FLP) in solution. The MTO/FLP catalyst is active in ring-opening metathesis polymerization of norbornene and in cross-metathesis of internal olefins under mild conditions. ESI-MS and NMR studies found that MTO is deprotonated in the presence of the FLP to yield a rhenium-methylidene species. While this initially activated methylidene eluded detection, spraying reaction mixtures with structurally constrained olefins in ESI-MS allowed for the detection of on-cycle rhenium-alkylidene species. Time-course measurements showed that the modest catalytic activity could be attributed to a rapid catalyst deactivation step. One possible deactivation pathway was identified to be a second deprotonation step of the metathesis-active methylidene, yielding a rhenium-methylidyne. Kinetic experiments have shown that it can be reactivated for olefin metathesis by protonation in solution. Additionally, several irreversible catalyst deactivation pathways leading to permanently deactivated catalyst species are hypothesized. We propose that the MTO/FLP system constitutes a homogeneous model system for the heterogeneous MTO catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


