基于膦酸盐的氮杂环配体用于低温、稳定地螯合药用稀土放射性金属和放射性氟化反应
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
氟(18F)、钪(43/44Sc、47Sc)、镥(177Lu)和钇(86Y、90Y)的放射性同位素具有衰变特性,非常适合与肽和抗体片段等小型生物制剂一起进行靶向核成像和治疗。然而,在温和的条件下将这些放射性核素引入放射性药物以获得惰性体内复合物的单分子策略还非常缺乏。在这里,我们介绍了 H4L2 和 H4L3,这是两种带有单一膦酸盐官能团的小腔大环螯合剂结构异构体。我们采用电位计和分光光度法测定了 H4L2 和 H4L3′在生理相关条件下与不同大小的金属(Sc3+、Lu3+ 和 Y3+)形成单一 [M(L)]- 物种的能力。核磁共振光谱和密度泛函理论(DFT)计算表明,在 Sc3+/Lu3+/Y3+ 系列中,H4L2 和 H4L3′的内球水合作用发生了变化。用 18F、44Sc、177Lu 和 86Y 进行的放射化学标记实验表明,H4L2 在室温下能以较高的表观摩尔活度(AMA,462 mCi/μmol)选择性地螯合放射性钪,而放射性氟化仍无法进入。与此相反,H4L3 可在室温下放射化 44Sc、177Lu 和 86Y(AMA:96-275 mCi/μmol),并通过 Sc-18F 方法结合 18F,形成 [18F][ScF(L3)]2-。注射后 1 小时的体内生物分布分析证实了 H4L3 的广泛用途:所有四种放射化学复合物都能清除靶外器官,并在尿液代谢物分析中保持 98% 的完整度。室温放射化学标记与简便的 18F 结合可产生体内兼容的复合物,其范围超过了临床金标准螯合剂 DOTA 和之前报道的无环螯合剂,这使得 H4L3 在未来的放射性药物应用中大有可为。本文章由计算机程序翻译,如有差异,请以英文原文为准。
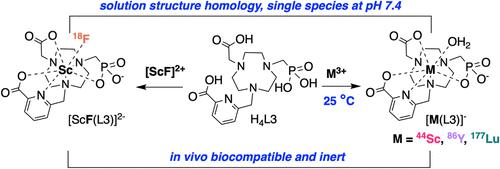
Phosphonate-Based Aza-Macrocycle Ligands for Low-Temperature, Stable Chelation of Medicinally Relevant Rare Earth Radiometals and Radiofluorination
Radioisotopes of fluorine (18F), scandium (43/44Sc, 47Sc), lutetium (177Lu), and yttrium (86Y, 90Y) have decay properties ideally suited for targeted nuclear imaging and therapy with small biologics, such as peptides and antibody fragments. However, a single-molecule strategy to introduce these radionuclides into radiopharmaceuticals under mild conditions to afford inert in vivo complexes is critically lacking. Here, we introduce H4L2 and H4L3, two small-cavity macrocyclic chelator structural isomers bearing a single phosphonate functional group. Potentiometry and spectrophotometry were employed to determine H4L2 and H4L3′s ability to form a single [M(L)]− species with metals of different sizes (Sc3+, Lu3+, and Y3+) under physiologically relevant conditions. NMR spectroscopy and density functional theory (DFT) calculations suggest modulation of H4L2 and H4L3′s inner-sphere hydration across the Sc3+/Lu3+/Y3+ series. Radiochemical labeling experiments with 18F, 44Sc, 177Lu, and 86Y reveal that H4L2 selectively chelates radioscandium at room temperature with high apparent molar activity (AMA, 462 mCi/μmol), while radiofluorination remains inaccessible. In contrast, H4L3 enables room temperature radiochelation 44Sc, 177Lu, and 86Y (AMA: 96–275 mCi/μmol) and incorporates 18F via the Sc–18F methodology to form [18F][ScF(L3)]2–. In vivo biodistribution analysis at 1 h postinjection confirms the broad utility of H4L3: all four radiochemical complexes clear off-target organs and remain >98% intact in urine metabolite analyses. The scope of room temperature radiochemical labeling, paired with facile 18F incorporation, to afford in vivo compatible complexes exceeds the clinical gold standard chelator DOTA and previously reported acyclic chelators, rendering H4L3 promising for prospective radiopharmaceutical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


