通过化学酶促缩聚合成半芳香 AA/BB 型聚酰胺
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
在水性缓冲液中使用木瓜蛋白酶,通过二胺和二酯型芳香族单体的化学酶促缩聚反应,合成了主链中含有芳香族分子的多肽 AA/BB 型聚酰胺。为了减轻木瓜蛋白酶对芳香族单元底物识别率低的问题,用甘氨酸对芳香族二胺(如 1,3-苯二胺(Pda)、2,4-二氨基苯甲醚(Dan)和 2,4-二氨基苯酚(Dap))和芳香族二元酸(如 5-氨基间苯二甲酸(Aip))进行了修饰。二胺单体 GlyPdaGly、GlyDanGly 和 GlyDapGly 以及二酯单体 GlyAipGly 在木瓜蛋白酶的作用下进行缩聚、形成 AA/BB 型聚酰胺聚(GlyPdaGly-alt-GlyAipGly)(AP(GG))、聚(GlyDanGly-alt-GlyAipGly)(AP(GG)OMe)和聚(GlyDapGly-alt-GlyAipGly)(AP(GG)OH)。通过傅立叶变换红外光谱(FT-IR)和广角 X 射线衍射(WAXD)对这些聚酰胺进行的结构分析表明,AP(GG)系列在很大程度上是无定形的,这是由于主链中含有芳香族分子而导致 PolyGly 的氢键减少所致。AP(GG) 系列的热分析表明其具有较高的热稳定性和热塑性,这是由苯环的刚性结构和聚酰胺的无定形结构造成的。AP(GG) 、AP(GG)OMe 和 AP(GG)OH 的缩聚后产物通过 Aip 氨基的酰胺化作用形成了超支化/网状结构。值得注意的是,化学酶聚合和后缩聚提供了具有高热稳定性的肽基半芳香族聚酰胺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
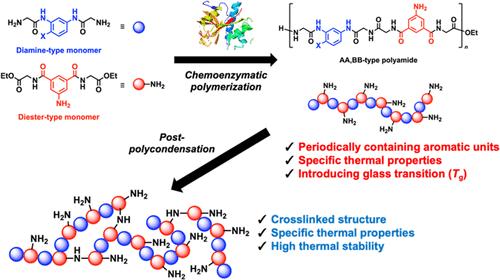
Synthesis of Semiaromatic AA/BB-Type Polyamides via Chemoenzymatic Polycondensation
Polypeptide-based AA/BB-type polyamides containing aromatic moieties in the main chains were synthesized via chemoenzymatic polycondensation of diamine and diester-type aromatic monomers through the use of papain in aqueous buffers. To mitigate the low substrate recognition of papain by aromatic units, aromatic diamines, such as 1,3-phenylenediamine (Pda), 2,4-diaminoanisole (Dan) and 2,4-diaminophenol (Dap), and aromatic diacids, such as 5-aminoisophthalic acid (Aip), were modified with glycine. The diamine monomers GlyPdaGly, GlyDanGly and GlyDapGly and diester monomer GlyAipGly were used for polycondensation in the presence of papain, resulting in the formation of the AA/BB-type polyamides poly(GlyPdaGly-alt-GlyAipGly) (AP(GG)), poly(GlyDanGly-alt-GlyAipGly) (AP(GG)OMe) and poly(GlyDapGly-alt-GlyAipGly) (AP(GG)OH). Structural analysis of the polyamides via Fourier transform infrared (FT-IR) spectroscopy and wide-angle X-ray diffraction (WAXD) revealed that the AP(GG) series was largely amorphous, resulting from a reduction in the hydrogen bonding of PolyGly due to the inclusion of aromatic moieties in the main chain. Thermal analysis of the AP(GG) series revealed high thermal stability and thermoplasticity, which are caused by the rigid structure of the benzene ring and the amorphous structure of the polyamides. The postpolycondensation products of AP(GG), AP(GG)OMe and AP(GG)OH formed hyperbranched/networked structures via amidation of Aip amines. Notably, chemoenzymatic polymerization followed by postpolycondensation provided peptide-based semiaromatic polyamides with high thermal stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


