帕金森病中 CHCHD2 的神经保护作用:洞察与 GPX4 相关的铁氧化途径
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
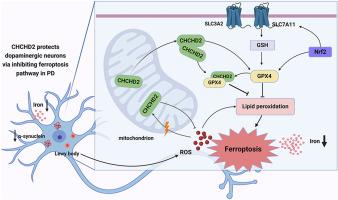
帕金森病(Parkinson's disease,PD)是第二大神经退行性疾病,其发病机制涉及线粒体功能障碍、氧化应激和铁变态反应。遗憾的是,目前还没有有效的干预措施来减缓帕金森病的进展。据报道,线粒体蛋白含卷曲螺旋-卷曲-螺旋结构域 2(CHCHD2)与神经退行性病变有牵连,可作为帕金森病的生物标志物,它对氧化应激有神经保护作用,但其中潜在的分子机制仍难以捉摸。在这项研究中,我们通过基于串联质量标记(TMT)的蛋白质组分析,发现了CHCHD2保护神经元细胞免受氧化应激的关键机制,其中铁蛋白沉积途径发挥了关键作用。据观察,过表达 CHCHD2 可提高 PD 细胞的存活率,降低脂质过氧化和活性氧(ROS)的水平,并上调铁氧化负调控蛋白谷胱甘肽过氧化物酶 4(GPX4)的表达。相反,敲除 CHCHD2 会导致细胞活力降低、脂质过氧化反应升高和 GPX4 表达减少。此外,CHCHD2 的过表达可改善运动功能障碍,降低α-突触核蛋白水平,减轻帕金森病小鼠黑质和纹状体中多巴胺能(DA)神经元的缺失。重要的是,我们的研究表明,CHCHD2对帕金森病中铁细胞凋亡的抑制作用与GPX4信号通路有关。总之,我们的研究阐明了CHCHD2在调节与GPX4相关的帕金森病铁氧化途径中的神经保护作用,为未来帕金森病药物的开发和治疗提供了新的靶点和思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Neuroprotective Role of CHCHD2 in Parkinson's Disease: Insights into the GPX4-Related Ferroptosis Pathway
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease, characterized by pathogenesis involving mitochondrial dysfunction, oxidative stress, and ferroptosis. Unfortunately, there are currently no effective interventions to slow down the progression of PD. The mitochondrial protein coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2), which is implicated in neurodegeneration and serves as a biomarker for PD, has been reported to have neuroprotective effects against oxidative stress, but the potential molecular mechanisms involved remain elusive. In this study, we uncovered a critical mechanism by which CHCHD2 protected neuronal cells against oxidative stress with the ferroptosis pathway playing a pivotal role, as determined through tandem mass tags (TMT)-based proteomic analysis. The overexpression of CHCHD2 was observed to enhance cell viability, reduce levels of lipid peroxidation and reactive oxygen species (ROS), and upregulate the expression of the ferroptosis negative regulatory protein Glutathione peroxidase 4 (GPX4) in PD cells. Conversely, CHCHD2 knockdown led to reduced cell viability, elevated lipid peroxidation, and a decreased expression of GPX4. Additionally, CHCHD2 overexpression ameliorated motor function impairment, reduced α-synuclein levels, and mitigated dopaminergic (DA) neuron loss in the substantia nigra and striatum of PD mice. Importantly, we show that the inhibitory effect of CHCHD2 on ferroptosis in PD is related to the GPX4 signaling pathway. In summary, our study elucidates the neuroprotective role of CHCHD2 in regulating the GPX4-related ferroptosis pathway in PD, providing new targets and ideas for future PD drug development and therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


