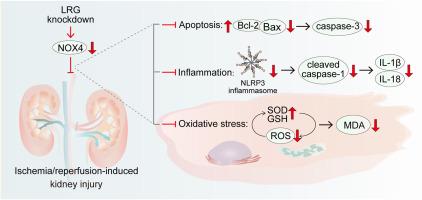
敲除富亮氨酸α-2-糖蛋白1可抑制NOX4介导的细胞凋亡、炎症和氧化应激,从而减轻肾缺血再灌注损伤。
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
肾缺血再灌注(I/R)损伤主要导致急性肾损伤。富亮氨酸α-2-糖蛋白 1(LRG)在肾脏 I/R 损伤后的小鼠肾脏组织中上调。然而,它在肾脏 I/R 损伤中的作用尚未完全阐明。通过单侧肾蒂夹闭和再灌注,构建了肾脏I/R损伤小鼠模型。与接受假手术的小鼠相比,接受 I/R 手术的小鼠表现出肾功能损伤和 LRG 蛋白表达增加。尾静脉注射携带 shLRG 的慢病毒可降低肾脏 I/R 损伤引起的 caspase-3 活性、IL-1β 和 IL-18 浓度以及 ROS 生成的增加。此外,shRNA介导的HK-2细胞LRG敲除保护了H/R诱导的细胞损伤。LRG能上调NADPH氧化酶4(NOX4)的表达。我们还检测了肾脏I/R手术小鼠肾组织和H/R处理的HK-2细胞中NOX4表达的增加。NOX4 的过表达逆转了 LRG 敲除对 H/R 引起的 HK-2 细胞损伤的抑制作用。总之,我们的研究结果表明,LRG敲除可降低NOX4的表达,从而通过抑制细胞凋亡、炎症和氧化应激减轻肾脏I/R损伤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Knockdown of Leucine-rich alpha-2-glycoprotein 1 alleviates renal ischemia-reperfusion injury by inhibiting NOX4-mediated apoptosis, inflammation, and oxidative stress
Renal ischemia-reperfusion (I/R) injury leads mainly to acute kidney injury. Leucine-rich alpha-2-glycoprotein 1 (LRG) is upregulated in kidney tissues of mice after renal I/R injury. However, its role in renal I/R injury has not been fully elucidated. A mouse model of renal I/R injury was constructed by unilateral renal pedicle clamping and reperfusion. Mice undergoing I/R procedures exhibited renal function impairment and increased LRG protein expression compared with mice receiving sham operations. Tail vein injection with lentivirus carrying shLRG decreased renal I/R injury-induced increase in caspase-3 activity, IL-1β and IL-18 concentrations, and ROS production. Furthermore, shRNA-mediated LRG knockdown in HK-2 cells protected against H/R-induced cell damage. LRG could upregulate the expression of NADPH oxidase 4 (NOX4). We also determined the increased NOX4 expression in kidney tissues of renal I/R-operated mice and H/R-treated HK-2 cells. NOX4 overexpression reversed the inhibitory role of LRG knockdown in HK-2 cell damage caused by H/R. Collectively, our findings demonstrate that LRG knockdown decreases the NOX4 expression, thereby alleviating renal I/R injury by inhibiting cell apoptosis, inflammation, and oxidative stress.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


