坏死样细胞死亡过程中白细胞介素-1α的释放会产生髓系驱动的免疫抑制,从而限制抗肿瘤免疫力
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
坏死可以促进抗原特异性免疫反应,这表明诱导坏死是一种治疗癌症的方法。在这里,我们试图确定免疫激活的机制,但发现在乳腺癌或肺癌的遗传和植入模型中,坏死介质RIPK3和MLKL对肿瘤生长是不可或缺的。令人惊讶的是,在已确立的乳腺肿瘤中诱导坏死生成会产生一种骨髓抑制性微环境,从而抑制 T 细胞功能、促进肿瘤生长并降低存活率。这依赖于死亡细胞释放的核警报素白细胞介素-1α(IL-1α)。重要的是,IL-1α会在化疗期间释放,而靶向这一分子可降低肿瘤髓系细胞的免疫抑制能力,促进CD8+ T细胞的募集和效应功能。在临床前模型中,中和 IL-1α 可增强单药紫杉醇或与 PD-1 阻断剂联合治疗的疗效。在几种实体恶性肿瘤中,低IL1A水平与患者的预后相关,尤其是在接受化疗的患者中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
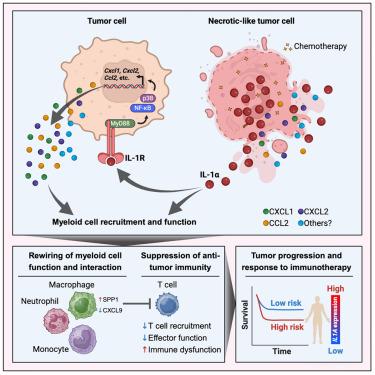
Interleukin-1α release during necrotic-like cell death generates myeloid-driven immunosuppression that restricts anti-tumor immunity
Necroptosis can promote antigen-specific immune responses, suggesting induced necroptosis as a therapeutic approach for cancer. Here we sought to determine the mechanism of immune activation but found the necroptosis mediators RIPK3 and MLKL dispensable for tumor growth in genetic and implantable models of breast or lung cancer. Surprisingly, inducing necroptosis within established breast tumors generates a myeloid suppressive microenvironment that inhibits T cell function, promotes tumor growth, and reduces survival. This was dependent upon the release of the nuclear alarmin interleukin-1α (IL-1α) by dying cells. Critically, IL-1α release occurs during chemotherapy and targeting this molecule reduces the immunosuppressive capacity of tumor myeloid cells and promotes CD8+ T cell recruitment and effector function. Neutralizing IL-1α enhances the efficacy of single agent paclitaxel or combination therapy with PD-1 blockade in preclinical models. Low IL1A levels correlates with positive patient outcome in several solid malignancies, particularly in patients treated with chemotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


