金催化剂在甘油选择性氧化过程中的开路电位
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
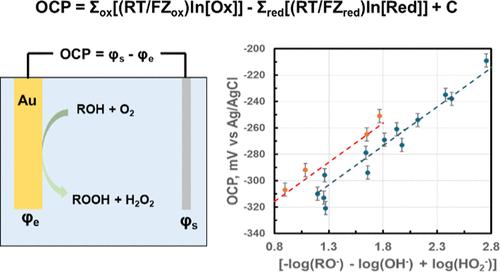
了解金属催化剂的电位与其热化学催化活性之间的关系,尤其是在水相环境中的关系,一直是人们非常感兴趣的问题。在文献中,开路电位与反应速率或反应中间产物的表面浓度存在相关性。在本研究中,我们测量了金纱布在碱性溶液中将甘油选择性氧化成甘油酸和过氧化氢过程中的开路电位(OCP)与氢氧化物和醇酸盐浓度的函数关系。研究发现,对金催化剂施加电位对反应速率没有影响。虽然 OCP 与反应速率之间似乎存在着大致的相关性,但数据与一个方程式的拟合效果更好,该方程式假定金属的电位(即 OCP)与溶液的电化学电位处于平衡状态,而溶液的电化学电位是由氧化性和还原性物种的热力学活动确定的。方程式为OCP = 常量 + Σox[(RT/FZox) ln[Ox]] - Σred[(RT/FZred) ln[Red]] 暗含了金金属作为探测电极的假设。我们进一步发现,这一方程式也适用于金催化的 H2O2 在碱性介质中的氧化/分解反应,也可能适用于甲酸脱氢反应。我们推测,OCP 与反应速率之间的明显相关性是由于反应速率与反应产物和/或反应物的浓度成正比,而反应产物和/或反应物的浓度决定了溶液的电化学势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Open Circuit Potential of a Au Catalyst during Selective Oxidation of Glycerol
It has been of great interest to understand the relationships between the potential of a metal catalyst and its thermochemical catalytic activity, especially in an aqueous phase environment. In the literature, there are correlations of open circuit potential with reaction rates or surface concentrations of the reaction intermediates. In this study, we measured the open circuit potential (OCP) of a Au gauze during selective oxidation of glycerol to glyceric acid and hydrogen peroxide in a basic solution as a function of hydroxide and alcoholate concentrations. It is found that applying a potential to the Au catalyst has no influence on the reaction rate. Although a rough correlation appears to exist between the OCP and reaction rate, the data are better fit to an equation which assumes that the potential of the metal (i.e., the OCP) is in equilibrium with the electrochemical potential of the solution, which is defined by the thermodynamic activities of the oxidizing and reducing species. The equation: OCP = constant + Σox[(RT/FZox) ln[Ox]] – Σred[(RT/FZred) ln[Red]] implicitly assumes that the Au metal functions as a probing electrode. It is further found that this equation also applies to the Au-catalyzed H2O2 oxidation/decomposition reaction in a basic medium and possibly to formic acid dehydrogenation. We postulate that the apparent correlation between OCP and reaction rate is due to the fact that the reaction rate is proportional to the concentrations of the reaction products and/or reactants that define the electrochemical potential of the solution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


