氢氧化镍沉淀的氧化还原过程及其碳热还原的研究
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
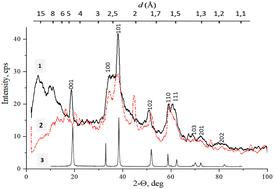
已知的化学生产纳米镍和微粒、镍氧化物和氢氧化物的方法大多使用各种还原剂和溶剂,而这些还原剂和溶剂通常对环境有毒。通常情况下,这些方法耗能、耗时长、步骤多,需要复杂的设备。因此,开发一种简单而 "绿色 "的工艺来合成含镍微粒(包括具有磁性的微粒)仍然是优先任务之一。本文提出了一种从含镍电解质水溶液中在镁粒子表面氧化-还原接触沉积氢氧化镍(II)纳米微粒的新物理化学方法。该方法基于以氢去极化为主的微galvanic电池形成的局部腐蚀。利用所提出的方法获得了氢氧化镍(II)样品,并利用扫描电镜研究了它们的形态,还利用 XRD 分析研究了它们的相组成。研究证明,所得到的 Ni(OH)2 颗粒的形状和结构取决于接触沉积条件:根据作为还原剂的镁颗粒的表面状态,既可以得到板状的 α/β-Ni(OH)2 颗粒,也可以得到具有不同结晶度的三维 β-Ni(OH)2 "花"。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigation of oxidation–reduction processes of nickel hydroxide precipitation and their carbothermical reduction
Most of the known methods for the chemical production of nickel nano- and microparticles, nickel oxides and hydroxides use various reducing agents and solvents, which are often toxic to the environment. As a rule, these methods are energy-consuming, lengthy and multi-stage, requiring complex equipment. Therefore, the development of a simple and “green” process for the synthesis of nickel-containing particles, including those with magnetic properties, remains one of the priority tasks. In this paper, a new physicochemical method for oxidation–reduction contact deposition of nickel(II) hydroxide nano–microparticles on the surface of magnesium particles from aqueous solutions of nickel-containing electrolyte is proposed. This method is based on the local corrosion of microgalvanic cells’ formation with predominant hydrogen depolarization. The proposed method was used to obtain nickel(II) hydroxide samples and study their morphology using SEM, as well as their phase composition using XRD analysis. It has been proven that the shape and structure of the resulting Ni(OH)2 particles depend on the contact deposition conditions: depending on the surface state of the magnesium particles as a reducing agent, it is possible to obtain both plate-shaped α/β-Ni(OH)2 particles and three-dimensional β-Ni(OH)2 “flowers” with different degrees of crystallinity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


