利用烷氧基烯催化的 BINAP-CuH 对映体选择性烯丙基化反应获得 1,2-辛-叔,仲-二醇
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们介绍了一种经济的方法,即使用 PMHS 作为氢化物源,通过 Cu-BINAP 催化,将烷氧基烯与一系列电子和结构不同的酮进行高对映选择性和非对映选择性还原偶联,从而得到 1,2-辛-叔,仲-二醇。这种还原偶联还被有效地应用于含有季中心的亲手性环酮的对映选择性去对称化,产量高且具有独有的非对映选择性。密度泛函理论(DFT)计算阐明,该反应是由一个动力学上有利的 "开放 "Z-烯酸铜-烷氧基烯构象促进的,其发生的吉布斯自由能垒(3.9 kcal mol-1)低于其对应的 E-烯酸铜构象,从而决定了立体选择性。随后,这种 Z-烯酸酯构象体与适当的亲核面同步,在酮加成过程中通过六元椅状过渡态在产物中实现目标的同步双向选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
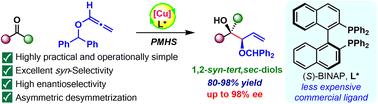
BINAP-CuH-catalysed enantioselective allylation using alkoxyallenes to access 1,2-syn-tert,sec-diols
Herein, we present an economical method for highly enantioselective and diastereoselective Cu-BINAP-catalysed reductive coupling of alkoxyallenes with a range of electronically and structurally diverse ketones to afford 1,2-syn-tert,sec-diols, using PMHS as the hydride source. This reductive coupling has also been efficiently employed in the enantioselective desymmetrization of prochiral cyclic ketones harboring quaternary centres, in high yields with exclusive diastereoselectivity. Density Functional Theory (DFT) calculations are used to elucidate that the reaction is facilitated by a kinetically favourable “open” Z-enolate copper–alkoxyallene conformer, occurring at a lower Gibbs free energy barrier (by 3.9 kcal mol−1) than its E-enolate counterpart, dictating the stereoselectivity. Subsequently, this Z-enolate conformer synchronizes with appropriate nucleophilic faces to achieve the targeted syn-diastereoselectivity in the product through six-membered chair-like transition states during ketone addition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


