与羧酸和硫代羧酸端基二元烷分子相连的量子点的光致发光切换
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
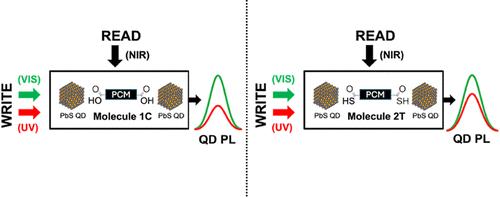
我们对比了与具有不同端基的光致变色二元蒽分子(4、4′-(1-环戊烯-1,2-二基)双[5-甲基-2-噻吩羧酸](1C)和 4,4′-(1-环戊烯-1,2-二基)双[5-甲基-2-噻吩硫代羧酸](2T)。我们的研究结果表明,与硫代羧酸端基交联的 QDs(2T)相比,与羧酸端基分子交联的 QDs(1C)表现出更大的光致发光强度变化。我们还证明,无论使用哪种分子,较小的量子点都能观察到更大的切换量。改变这些参数可以制造出具有可调 PL 变化的光开关。我们将这些观察结果与量子点和光致变色分子之间的 HOMO 能级差异联系起来。我们的研究结果表明了 QDs 的尺寸和链接配体的能级如何影响电荷隧道率,从而影响基于隧道的光开关的光致变色性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Photoluminescence Switching in Quantum Dots Connected with Carboxylic Acid and Thiocarboxylic Acid End-Group Diarylethene Molecules
We contrast the switching of photoluminescence (PL) of PbS quantum dots (QDs) cross-linked with photochromic diarylethene molecules with different end groups, 4,4′-(1-cyclopentene-1,2-diyl)bis[5-methyl-2-thiophenecarboxylic acid] (1C) and 4,4′-(1-cyclopentene-1,2-diyl)bis[5-methyl-2-thiophenethiocarboxylic acid] (2T). Our results show that the QDs cross-linked with the carboxylic acid end group molecules (1C) exhibit a greater amount of switching in photoluminescence intensity compared to QDs cross-linked with the thiocarboxylic acid end group (2T). We also demonstrate that regardless of the molecule used, greater switching amounts are observed for smaller quantum dots. Varying these parameters allows for the fabrication of photoswitches with tunable PL change. We relate these observations to the differences in the HOMO energy levels between the QDs and the photochromic molecules. Our findings demonstrate how the size of the QDs and the energy levels of the linker ligands influences the charge tunneling rate and thus the PL switching performance in tunneling-based photoswitches.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


