二炔烃的 N-定向双溴化反应使溴化 BN 嵌入多环芳烃成为可能
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发了二炔与 BBr3 的 N-定向 2 倍溴化反应,从而可以在温和的条件下,从容易获得的前体中获得新型的内部掺杂 BN 的多环芳烃。计算研究发现了三种潜在的反应机理,每种机理都涉及 BBr3 或 [BBr4]-,所有途径的活化障碍都很低(ΔG‡ < 16 kcal/mol)。生成的溴化产物可通过各种交叉耦合协议进一步官能化,从而合成出量子产率超过 90 的高发光发光体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
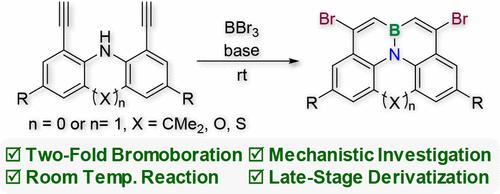
N-Directed Two-Fold Bromoboration of Diynes Enables Access to Brominated BN-Embedded PAHs
N-directed 2-fold bromoboration reactions of diynes with BBr3 have been developed, allowing the access to novel internally BN-doped polycyclic aromatic hydrocarbons from readily available precursors under mild conditions. Computational investigations identified three potential reaction mechanisms, each involving either BBr3 or [BBr4]−, with low activation barriers (ΔG‡ < 16 kcal/mol) for all pathways. The resulting brominated products can be further functionalized through various cross-coupling protocols, enabling the synthesis of highly luminescent emitters with quantum yield exceeding 90.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


