NAMPT 的隐性磷酸核糖酶活性限制了病毒蛋白在病毒中的结合
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
作为强制性细胞内病原体,病毒会激活宿主的代谢酶,以提供支持后代产生的中间产物。烟酰胺磷酸核糖转移酶(NAMPT)是烟酰胺腺嘌呤二核苷酸(NAD+)合成的限速酶,它是一种干扰素诱导蛋白,能通过未知机制抑制多种 RNA 和 DNA 病毒的复制。在这里,我们发现 NAMPT 通过其磷酸核糖水解酶(phosphoribosyl-hydrolase)活性阻碍病毒蛋白在病毒中的结合,从而限制了单纯疱疹病毒 1 型(HSV-1)的复制,而这与 NAMPT 在 NAD+ 合成中的作用无关。对 HSV-1 感染细胞进行的蛋白质组学分析确定了磷酸核糖基化的病毒结构蛋白,特别是糖蛋白和保护膜蛋白,这些蛋白在体外和细胞内被 NAMPT 去磷酸核糖基化。携带磷酸核糖基化抗性突变的嵌合型和重组型 HSV-1 表明,磷酸核糖基化促进了结构蛋白与 HSV-1 病毒的结合以及随后的病毒进入。缺失 NAMPT 会使小鼠极易感染 HSV-1。我们的工作描述了一种代谢酶在病毒感染和宿主防御中的额外酶活性,为研究蛋白质磷酸化在类人猿中的作用提供了一个系统。本文章由计算机程序翻译,如有差异,请以英文原文为准。
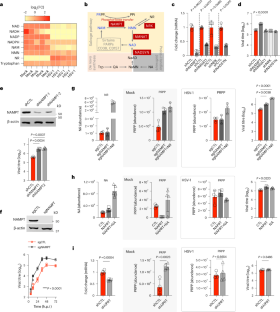

Cryptic phosphoribosylase activity of NAMPT restricts the virion incorporation of viral proteins
As obligate intracellular pathogens, viruses activate host metabolic enzymes to supply intermediates that support progeny production. Nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme of salvage nicotinamide adenine dinucleotide (NAD+) synthesis, is an interferon-inducible protein that inhibits the replication of several RNA and DNA viruses through unknown mechanisms. Here, we show that NAMPT restricts herpes simplex virus type 1 (HSV-1) replication by impeding the virion incorporation of viral proteins owing to its phosphoribosyl-hydrolase (phosphoribosylase) activity, which is independent of the role of NAMPT in NAD+ synthesis. Proteomics analysis of HSV-1-infected cells identifies phosphoribosylated viral structural proteins, particularly glycoproteins and tegument proteins, which are de-phosphoribosylated by NAMPT in vitro and in cells. Chimeric and recombinant HSV-1 carrying phosphoribosylation-resistant mutations show that phosphoribosylation promotes the incorporation of structural proteins into HSV-1 virions and subsequent virus entry. Loss of NAMPT renders mice highly susceptible to HSV-1 infection. Our work describes an additional enzymatic activity of a metabolic enzyme in viral infection and host defence, offering a system to interrogate the roles of protein phosphoribosylation in metazoans. The NAD+ synthesis enzyme NAMPT is shown to possess additional enzymatic activity as a phosphoribosylase, which restricts the virion incorporation of viral proteins and underpins its antiviral effect
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


