在重型再生障碍性贫血强化免疫抑制疗法中加入艾曲波帕,可帮助成年患者获得与儿科患者相似的疗效
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
摘要
再生障碍性贫血(AA)是一种骨髓无法产生足够血细胞的疾病[1]。对于不符合造血干细胞移植(HSCT)条件的重型再生障碍性贫血(SAA)患者,建议使用抗胸腺细胞球蛋白(ATG)和环孢素A(CsA)进行强化免疫抑制治疗(IST)[2]。然而,儿童单独使用 IST 的疗效要高于成人。最近,艾曲波帕(EPAG)被证明可增强 AA 治疗的血液学反应 [3,4]。IST + EPAG 治疗期间是否仍存在成人与儿童之间的差异仍不清楚。迄今为止,尚未对成人和儿童进行过直接比较。本研究旨在评估采用不同方案治疗的成人和儿童在疗效和存活率方面的差异。在单独接受 IST 治疗的患者中,成人在 12 个月时的完全应答率(CRR)低于儿童(31% vs. 48%,P = 0.048),但成人和儿童在 3、6 或 12 个月时的总应答率(ORR)没有差异(分别为 52% vs. 59%,P = 0.426;69% vs. 74%,P = 0.599;76% vs. 75%,P = 1.000)。与此相关的是,成人和儿童在 3 个月和 6 个月时的 CRR 没有差异(分别为 3% vs. 11%,P = 0.113;21% vs. 22%,P = 0.849)。成人的反应时间为4.3(IQR 2.9-6.3)个月,儿童为3.2(IQR 2.5-4.0)个月(P = 0.243);成人的CR时间为8.5(IQR 6.8-11.0)个月,儿童为7.5(IQR 6.0-10.5)个月(P = 0.113)。在 IST + EPAG 患者中,成人和儿童在 3 个月或 6 个月时的 ORR 没有差异(分别为 67% 对 64%,P = 0.868;83% 对 76%,P = 0.567),但成人在 12 个月时的 ORR 较高(89% 对 73%,P = 0.027)。两组患者在3、6和12个月时的CRR也没有差异(分别为13% vs. 21%,P = 0.229;25% vs. 38%,P = 0.124;54% vs. 50%,P = 0.614;图1)。成人出现反应的时间为 3.0(IQR 2.8-3.3)个月,儿童为 2.5(IQR 1.9-3.9)个月(P = 0.361)。此外,成人的 CR 时间为 6.0(IQR 3.0-8.3)个月,儿童为 3.9(IQR 2.5-5.7)个月(P = 0.478)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
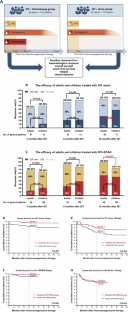

Adding eltrombopag to intensive immunosuppressive therapy for severe aplastic anaemia may help adult patients achieve outcomes similar to paediatric patients
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


