非小细胞肺癌患者在接受抗血管内皮生长因子受体2(VEGFR2)治疗前接受抗PD-L1治疗可获得更显著的临床疗效。
IF 4.8
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
摘要
目的:抗血管生成疗法和免疫检查点阻断疗法是目前治疗非小细胞肺癌的重要方法:抗血管生成疗法和免疫检查点阻断疗法是目前治疗非小细胞肺癌的重要方法。然而,这两种疗法的联合使用存在争议,很少有研究调查两种疗法的不同时间顺序对治疗效果的影响:方法:建立肿瘤小鼠模型,将小鼠分为四组,包括 AA-ICB 序列组、ICB-AA 序列组、同步组和对照组。采用免疫组化方法评估肿瘤微血管和 PD-L1 的表达。使用流式细胞术评估选定的免疫细胞群。元分析和临床信息用于阐明给药序列的临床效果:结果:我们发现抗PD-L1治疗后再进行抗血管内皮生长因子受体2治疗对肿瘤生长的抑制效果最好。抗血管生成治疗和免疫检查点阻断治疗的不同顺序导致肿瘤微环境中肿瘤微血管和免疫细胞群的比例不同。我们进一步发现,在抗血管内皮生长因子受体(anti-VEGFR)治疗前给予抗PD-L1可使肿瘤血管和CD8+T细胞浸润趋于正常,并减少肿瘤微环境中的免疫抑制细胞。随后的再移植实验证实了这一治疗策略的长期疗效。荟萃分析证实,晚期非小细胞肺癌患者在接受抗血管生成治疗或联合治疗前接受免疫治疗,疗效更佳:我们的研究表明,免疫检查点治疗后抗血管生成治疗的疗效优于同期治疗,而抗血管生成治疗后再进行免疫治疗并不比独立的单药治疗带来更显著的临床获益。本文章由计算机程序翻译,如有差异,请以英文原文为准。
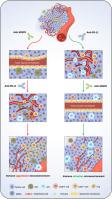
Sequential treatment of anti-PD-L1 therapy prior to anti-VEGFR2 therapy contributes to more significant clinical benefits in non-small cell lung cancer
Objective
Anti-angiogenic therapy and immune checkpoint blockade therapy are currently important treatments for non-small cell lung cancer. However, the combined use of the two therapies is controversial, and few studies have investigated the effects of different time sequences of the two therapies on treatment outcomes.
Methods
The tumor-bearing mouse model was established and the mice were divided into four groups, including AA-ICB sequence group, ICB-AA sequence group, synchronization group and the control group. Immunohistochemistry was used to assess tumor microvessels and PD-L1 expression. Selected immune cell populations were evaluated using flow cytometry. Meta-analysis and clinical information were used to elucidate the clinical effects of administration sequence.
Results
We found that anti-PD-L1 treatment followed by anti-VEGFR2 therapy exerts the best inhibitory effect on tumor growth. Different sequences of anti-angiogenic therapy and immune checkpoint blockade therapy resulted in different proportions of tumor microvessels and immune cell populations in the tumor microenvironment. We further revealed that the administration of anti-PD-L1 before anti-VEGFR brought more normalized tumor blood vessels and CD8+T cell infiltration and reduced immunosuppressive cells in the tumor microenvironment. Subsequent re-transplantation experiments confirmed the long-term benefits of this treatment strategy. The meta-analysis reinforced that immunotherapy prior to anti-angiogenic therapy or combination therapy have better therapeutic effects in advanced non-small cell lung cancer.
Conclusion
Our study demonstrated that the therapeutic effect of anti-angiogenic treatment after immune checkpoint therapy was superior to that of concurrent therapy, whereas anti-angiogenic therapy followed by immunotherapy did not bring more significant clinical benefits than independent monotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


