雷尔兴奋岛:绘制内侧岛叶皮层的谷氨酸能(vGlut1+ 和 vGlut2+)连接图。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
岛叶皮层是大脑皮层中一个功能多样、联系丰富的区域,在外部刺激和内部信号的神经整合中起着关键作用。通过庞大的传入和传出连接网络,小鼠岛叶可简化为前部、内侧和后部。在这里,我们重点讨论内侧亚区,这个曾经被忽视的区域最近因参与一系列行为而受到关注。虽然内侧岛叶皮层神经元的连接已被确定,但其准确的谷氨酸能表型仍未确定(通常是通过存在亚型的囊泡谷氨酸转运体来定义)。因此,我们结合 Cre 基因敲入小鼠品系和腺相关病毒追踪,以区分该亚区域神经元输入和输出中两种主要的谷氨酸囊泡转运体 1 型(vGlut1)和 2 型(vGlut2)的表达情况。我们的研究结果表明,内侧岛叶在整个神经轴中有大量同时表达 vGlut1 和 vGlut2 的谷氨酸传出。相比之下,我们观察到的谷氨酸能输入的数量更为保守,只有来自下丘脑和丘脑区域的 vGlut2+ 投射。总之,我们证明了这个岛状亚区的vGlut1-和vGlut2-表达网络具有不同的连接模式,包括更多的vGlut1+纤维支配下丘脑区域和扩展的杏仁核。这些发现有助于深入了解该区域独特的化学结构,从而有助于进一步研究内侧岛叶在复杂行为中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
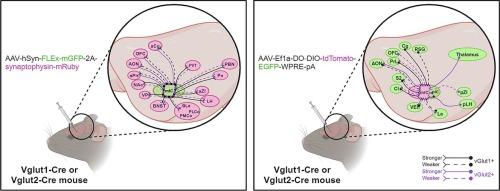
An Island of Reil excitation: Mapping glutamatergic (vGlut1+ and vGlut2+) connections in the medial insular cortex
The insular cortex is a multifunctional and richly connected region of the cerebral cortex, critical in the neural integration of external stimuli and internal signals. Well-served for this role by a large network of afferent and efferent connections, the mouse insula can be simplified into an anterior, medial and posterior portion. Here we focus on the medial subregion, a once over-looked area that has gained recent attention for its involvement in an array of behaviours. Although the connections of medial insular cortex neurons have been previously identified, their precise glutamatergic phenotype remains undefined (typically defined by the presence of the subtype of vesicular glutamate transporters). Hence, we combined Cre knock-in mouse lines and adeno-associated viral tracing to distinguish between the expression of the two major vesicular glutamate transporters, type 1 (vGlut1) and 2 (vGlut2), in the subregion’s neuronal inputs and outputs. Our results determined that the medial insula has extensive glutamatergic efferents expressing both vGlut1 and vGlut2 throughout the neuraxis. In contrast, a more conservative number of glutamatergic inputs were observed, with exclusively vGlut2+ projections received from hypothalamic and thalamic regions. Taken together, we demonstrate that vGlut1- and vGlut2-expressing networks of this insular subdivision have distinct connectivity patterns, including a greater abundance of vGlut1+ fibres innervating hypothalamic regions and the extended amygdala. These findings provide insight into the distinct chemo-architecture of this region, which may facilitate further investigation into the role of the medial insula in complex behaviour.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


