氧化锆支撑金纳米粒子催化氧气和甲酸生成过氧化氢过程中氧空位和水的作用
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
过氧化氢(H2O2)不仅是一种广泛应用于工业领域的清洁氧化剂,也是一种前景广阔的能源载体。人们强烈要求开发一种环境友好且可持续的方法来生产 H2O2,以替代目前耗能的蒽醌自氧化工艺。我们最近发现,在常温常压下,富含氧空位(Ov)或表面富含 OH 的金纳米粒子负载 ZrO2(Au/ZrO2)比富含 Ov 或表面 OH 的 Au/ZrO2 在从 O2 和 HCOOH 生成 H2O2 方面表现出更高的催化活性。为了弄清原因,我们通过密度泛函理论计算评估了可能的反应途径。结果表明,在 Au NP-ZrO2(Ov)-液三相界面附近,由于 Ov 和水的作用,一个 Langmuir-Hinshelwood 型反应通过双电子氧还原反应(2e-ORR)生成 H2O2。另一方面,在缺乏 Ov 或水的情况下,H2O 会优先通过 4e--ORR 生成。在氧化锆表面靠近金纳米粒子处产生的 Ov 通过电子传递从缺陷衍生的中隙水平经金纳米粒子传导至 O2,从而诱导 O2 的高效还原活化。另一方面,H2O优先吸附在Ov位点上,抑制了O2的O-O键裂解,导致4e--ORR。这项研究为水性介质中金/金属氧化物催化 ORR 的机理提供了重要的见解,而此前人们对这一机理知之甚少。本文章由计算机程序翻译,如有差异,请以英文原文为准。
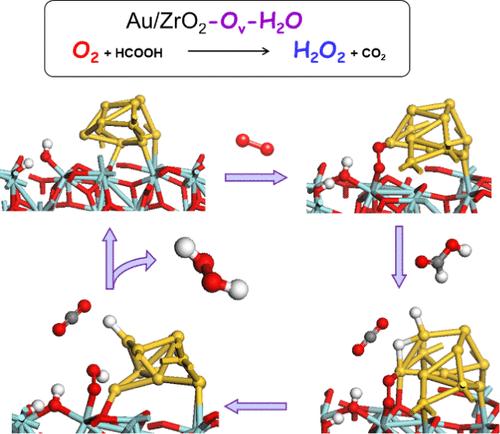
Role of Oxygen Vacancies and Water in Hydrogen Peroxide Production from Oxygen and Formic Acid Catalyzed by Zirconia-Supported Gold Nanoparticle
Hydrogen peroxide (H2O2) is not only a clean oxidant widely used in industry but also a promising energy carrier. There is a strong demand to develop an environmentally friendly and sustainable method for producing H2O2 as an alternative to the current energy-consuming anthraquinone autoxidation process. We recently found that Au nanoparticle-loaded ZrO2 (Au/ZrO2) rich in oxygen vacancy (Ov) or surface OH-rich exhibits much higher catalytic activity than Au/ZrO2 poor in Ov or surface OH for the production of H2O2 from O2 and HCOOH at ambient temperature and pressure. To clarify the reason, possible reaction pathways were evaluated by density functional theory calculations. The results indicated that a Langmuir–Hinshelwood-type reaction proceeds near the Au NP-ZrO2(Ov)-liquid three-phase interfaces to yield H2O2 via the two-electron oxygen reduction reaction (2e–-ORR) due to the cooperation of Ov and water. On the other hand, H2O is preferentially formed via 4e–-ORR when either Ov or water is lacking. The Ov generated on the ZrO2 surface near the Au NPs induces the efficient reductive activation of O2 by electron transfer from the defect-derived midgap levels to O2 through Au NPs. On the other hand, the preferential adsorption of H2O on the Ov sites inhibits the O–O bond cleavage of O2 leading to 4e–-ORR. This study provided important insight into the mechanism of the Au/metal oxide-catalyzed ORR in an aqueous medium, which was previously poorly understood.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


