氯仿过饱和形成有序氯层
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
离子在金属表面的吸附是改变其电子结构从而调整其反应活性的一种有效方法。一个突出的例子就是 Ag(111) 上的氯。我们利用低温扫描隧道显微镜研究了过饱和状态下氯仿在 Ag(111) 上的室温吸附所产生的氯,以及它从单个单体到全层所形成的结构。在超高真空条件下进行低温吸附后,利用温度编程解吸和 X 射线光电子能谱对数据进行了补充。考虑到分散力的密度泛函理论(DFT)计算为数据解释提供了支持。在低氯覆盖率条件下,每个氯都会局部改变表面的电子结构。由吸附的氯引起的局部环境改变为其附近的其他氯创造了优先吸附位点,从而稳定了 Ag(111) 上的扩展氯结构。寡聚体的形成会导致电荷转移的距离协同效应,从而对表面电子结构产生影响,这种影响超出了单个氯的变化。在中等氯覆盖率的情况下,氯形成蜿蜒的链,原子以不同的氯-氯距离交替吸附在 hcp 和 fcc 中空位点上。一维结构在中间覆盖率时转化为开放网络,在饱和覆盖率时转化为二维六边形上层结构。DFT 计算表明,随着氯原子在中等和高覆盖率时相邻得更近,从表面提取到氯原子的电荷密度以及氯原子和银原子之间的相互作用都得到了改善。本文章由计算机程序翻译,如有差异,请以英文原文为准。
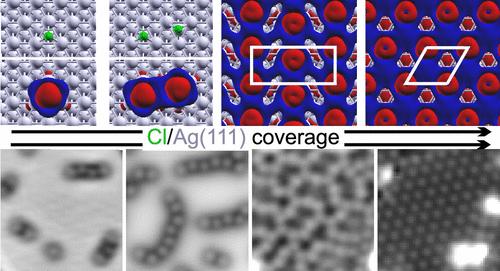
Ordered Chlorine Layer Formation from a Supersaturation of Chloroform
The adsorption of ions on metallic surfaces is a powerful method to alter their electronic structure and thus tune their reactivity. A prominent example is chlorine on Ag(111). We investigate chlorine created by the room-temperature adsorption of chloroform on Ag(111) at supersaturation and the structures it forms from individual monomers to a full layer by using low-temperature scanning tunneling microscopy. The data is supplemented by temperature-programmed desorption and X-ray photoelectron spectroscopy after low-temperature adsorption under ultrahigh-vacuum conditions. Data interpretation is supported by density functional theory (DFT) calculations that account for dispersion forces. At low chlorine coverages, each chlorine locally alters the electronic structure of the surface. The adsorbed chlorine-induced local environment modification thereby creates preferential adsorption sites for other chlorines in their vicinity, stabilizing extended chlorine structures on Ag(111). Oligomer formation leads to distance-dependent cooperative effects of the charge transfer and thus impacts the electronic structure of the surface beyond the change by individual chlorines. At intermediate chlorine coverage, chlorine forms meandering chains with atoms adsorbed in alternating hcp and fcc hollow sites at distinct chlorine–chlorine distances. The one-dimensional structures convert to an open network at intermediate coverages and a two-dimensional hexagonal superstructure at saturation coverage. The DFT calculations suggest that the charge density extracted from the surface into the chlorines and the interaction between chlorine and silver atoms is improved as chlorines are adjoined closer at intermediate and high coverages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


