氧化还原活性硼烷基二膦活化氨的 N-H 键
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在这项工作中,我们报告了硼酰基二膦 1-PtBu2-2-PiPr2-closo-C2B10H10 (1) 在室温下激活氨的 N-H 键,从而形成齐聚物 7-P(NH2)tBu2-10-P(H)iPr2-nido-C2B10H10(2)。与其他需要通过几何限制来增强亲电性的磷基消旋体不同,这种主族体系中的新键活化模式是基于富电子的三叉膦中心与电子接受硼烷簇之间的合作。在许多其他金属基和无金属体系中,碳化二膦 1 对气态氨或水态氢氧化铵的 N-H 键活化是一个例外,其活化过程对空气和水都没有影响。通过 DFT 方法对氨活化的机理细节进行了计算探索,结果表明氨是由膦中心亲电活化的。这一过程由硼簇的还原驱动,随后是氨辅助的去质子化和质子转移。随后,2 和 TEMPO 发生反应,导致所有 N-H 和 P-H 键断裂,形成由阴离子簇 N(7-PtBu2-8-PiPr2)-nido-C2B9H10 支持的环状磷硒阳离子 (3)。本文所报道的转化是通过无金属体系促进三重氢原子抽取实现氨氧化的首个实例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
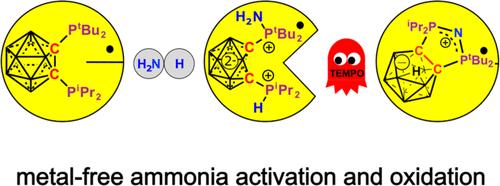
N–H Bond Activation of Ammonia by a Redox-Active Carboranyl Diphosphine
In this work, we report the room-temperature N–H bond activation of ammonia by the carboranyl diphosphine 1-PtBu2-2-PiPr2-closo-C2B10H10 (1) resulting in the formation of zwitterionic 7-P(NH2)tBu2-10-P(H)iPr2-nido-C2B10H10 (2). Unlike the other phosphorus-based ambiphiles that require geometric constraints to enhance electrophilicity, the new mode of bond activation in this main-group system is based on the cooperation between electron-rich trigonal phosphine centers and the electron-accepting carborane cluster. As an exception among many other metal-based and metal-free systems, the N–H bond activation of gaseous ammonia or aqueous ammonium hydroxide by carboranyl diphosphine 1 proceeds with tolerance of air and water. Mechanistic details of ammonia activation were explored computationally by DFT methods, demonstrating an electrophilic activation of ammonia by the phosphine center. This process is driven by the reduction of the boron cluster followed by an ammonia-assisted deprotonation and proton transfer. A subsequent reaction of 2 and TEMPO results in the cleavage of all N–H and P–H bonds with the formation of a cyclic phosphazenium cation supported by an anionic cluster N(7-PtBu2-8-PiPr2)-nido-C2B9H10 (3). Transformations reported herein represent the first example of ammonia oxidation via triple hydrogen atom abstraction facilitated by a metal-free system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


