使用自由基-极性共轭试剂对羧酸进行同系化反应
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在底物上添加一个碳原子的有机分子的同源物在药物发现中非常有用,可以获得具有改进特性的化合物,否则会给合成带来挑战。羧酸存在于许多生物活性分子中,是化学合成中广泛使用的基本成分,但它们的直接同源化还不为人所知。这种有价值的转化目前需要实施多步过程,需要使用羧酸衍生物而不是原生底物,而且通常涉及高活性和有毒试剂。在此,我们报告了首次在温和条件下使用新型、台式稳定的(1-磷酰)乙烯基磺酸试剂直接从原生羧酸进行一步同源转化的情况。这一策略被广泛应用于脂肪族羧酸构筑基块和与生物相关的复杂分子,一步即可获得一系列酯、酰胺和羧酸同系物。(1-磷酰)乙烯基磺酸试剂参与了以自由基链转移或有机光氧化催化为特征的互补同源化方案,并引入了一种新的合成物--二端丙烯酰自由基,以实现分子多样化。我们预计,这一策略解决了有机合成中一个长期存在的难题,通过快速合成多样化的同系物,将加速药物的发现。本文章由计算机程序翻译,如有差异,请以英文原文为准。
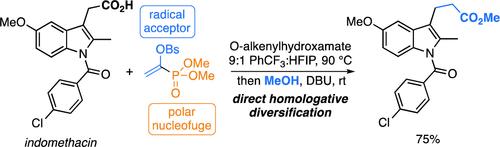
Homologation of Carboxylic Acids Using a Radical-Polar Conjunctive Reagent
Homologations of organic molecules that add a carbon atom to the substrate are useful in drug discovery to access compounds with improved properties that otherwise present a synthetic challenge. Carboxylic acids are present in many bioactive molecules and are widely available building blocks for chemical synthesis, yet their direct homologation is unknown. This valuable transformation currently necessitates implementation of multistep processes that require the use of carboxylic acid derivatives rather than the native substrates, and commonly involves highly reactive and toxic reagents. Herein, we report the first one-step homologation directly from native carboxylic acids using a novel, bench-stable (1-phosphoryl)vinyl sulfonate reagent under mild conditions. This strategy was applied to a wide range of aliphatic carboxylic acid building blocks and biologically relevant complex molecules to access an array of ester, amide, and carboxylic acid homologues in a single step. The (1-phosphoryl)vinyl sulfonate reagent participates in complementary homologation protocols featuring either radical-chain transfer or organic photoredox catalysis and introduces a new synthon, the distonic acylium radical, for molecular diversification. We anticipate this strategy, which addresses a long-standing challenge in organic synthesis, will expedite drug discovery by enabling the rapid synthesis of diversified homologues.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


