一种基于多肽核酸/多肽共聚物的多功能双模生物传感器,具有巨噬细胞搭便车功能,可用于增强肿瘤成像和尿液分析
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
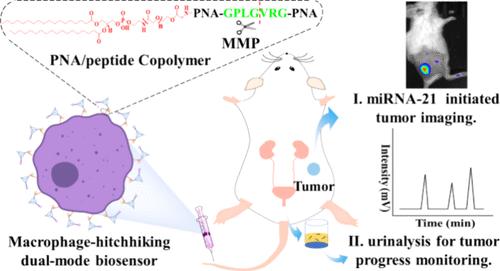
生物传感器能够通过活体液体活检成像诊断肿瘤,但面临着向肿瘤部位传输效率低和缺乏可靠的肿瘤相关生物标志物的挑战。在此,我们构建了一种基于多功能肽核酸(PNA)/肽共聚物和DNA四面体的双模式生物传感器,用于肿瘤成像和尿液分析。该生物传感器可进入癌细胞,在肿瘤微环境中被基质金属蛋白酶(MMP)裂解后引发microRNA-21特异性催化发夹组装反应,MMP裂解产物被释放到血液中,然后被肾脏滤出。由于 PNA 是一种人工合成的 DNA 类似物,不会被核酸酶和蛋白酶降解,因此可作为一种可靠的人工合成生物标记物,并可通过高效液相色谱法轻松检测尿液中的 PNA。重要的是,该生物传感器搭载在巨噬细胞膜上,利用巨噬细胞主动归巢到肿瘤部位并浸润肿瘤的能力,实现了在肿瘤深度的高效传递。结果表明,该生物传感器的信号输出显著提高,通过尿液检测可以可靠地分辨出肿瘤体积仅为 30-40 立方毫米的小鼠。这种创新的巨噬细胞搭便车双模式生物传感器作为一种无创、便捷的肿瘤诊断和肿瘤进展评估工具,具有巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A Multifunctional Peptide Nucleic Acid/Peptide Copolymer-Based Dual-Mode Biosensor with Macrophage-Hitchhiking for Enhanced Tumor Imaging and Urinalysis
Biosensors are capable of diagnosing tumors through imaging in vivoor liquid biopsy, but they face the challenges of inefficient delivery into tumor sites and the lack of reliable tumor-associated biomarkers. Herein, we constructed a dual-mode biosensor based on a multifunctional peptide nucleic acid (PNA)/peptide copolymer and DNA tetrahedron for tumor imaging and urinalysis. The biosensor could enter the cancer cells to initiate a microRNA-21-specific catalytic hairpin assembly reaction after cleavage by matrix-metalloprotease (MMP) in the tumor microenvironment, and the MMP cleavage product was released into the bloodstream and then was filtered out by the kidney. As PNA was a synthetic DNA analogue that could not be degraded by nucleases and proteases, it could serve as a reliable synthetic biomarker and be easily detected by high-performance liquid chromatography in urine. Importantly, the biosensor was hitchhiked on the macrophage membrane to realize efficient delivery in the depth of tumor utilizing the macrophage ability of actively homing to the tumor site and infiltrating into the tumor. The results indicated that the signal output of the biosensor was improved remarkably and mice with a tumor volume as little as 30–40 mm3 could be reliably discriminated through urine assay. This innovative macrophage-hitchhiking dual-mode biosensor holds a great potential as a non-invasive and convenient tool for tumor diagnosis and tumor progression evaluation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


