Ru(II)-catalyzed regioselective oxidative Heck reaction with internal olefins that tolerated strongly coordinating heterocycles(Ru(II)催化的可容忍强配位杂环的内部烯烃的区域选择性氧化 Heck 反应
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
强配位杂环与内部烯烃的氧化 Heck 反应常常导致难以捉摸的反应性和区域选择性。在此,我们在 Ru(II) 催化下通过合理选择 X 型指导基团,实现了强配位杂环与立体要求苛刻的内部烯烃的区域选择性氧化 Heck 反应。据推测,立体要求苛刻的内部烯烃作为偶联伙伴的 "匹配/错配效应",以及随后动力学上有利的迈克尔加成或氧化芳香化作用,是促进所需的反应性和位点选择性的驱动力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
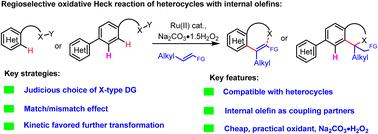

Ru(ii)-catalyzed regioselective oxidative Heck reaction with internal olefins that tolerated strongly coordinating heterocycles†
The oxidative Heck reaction of strongly coordinating heterocycles with internal olefins often led to elusive reactivity and regioselectivity. Herein, by judicious choice of X-type directing groups under Ru(II) catalysis, we achieved the regioselective oxidative Heck reaction of strongly coordinating heterocycles with sterically demanding internal olefins. It was postulated that the “match/mismatch effect” of sterically demanding internal olefins as coupling partners and subsequent kinetically favoured Michael addition or oxidative aromatization act as driving forces to facilitate the desired reactivity and site-selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


