揭示迈克尔-门顿速率定律的盲点:弛豫动力学在分子复合物形成中的作用。
IF 1.9
4区 数学
Q2 BIOLOGY
引用次数: 0
摘要
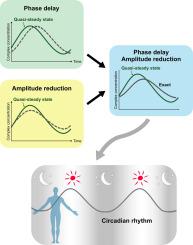
迈克尔-门顿(Michaelis-Menten)速率定律及其在生化速率过程建模中的修改已沿用了一个世纪,其假设条件是:与分子浓度变化的速度相比,相互作用分子的复合体浓度在每一时刻都迅速接近平衡(准稳态)。然而,对于具有瞬态或振荡动态的时变分子浓度,复合物曲线偏离准稳态的情况就变得非常重要。最近一种被称为有效时间延迟方案(ETS)的理论方法表明,分子复合物形成弛豫时间的延迟会导致准稳态假设的实质性破坏。在这里,我们系统地扩展了这一 ETS,并探究了松弛动力学在复合物形成过程中的综合作用。通过对有节奏的蛋白质-蛋白质和蛋白质-DNA 相互作用以及哺乳动物昼夜节律时钟的建模,我们的分析揭示了弛豫动力学在时间延迟之外的影响,它延伸到了对复合物浓度变化的抑制,与准稳态相比,振荡幅度减小了。有趣的是,时间延迟和振幅减小的综合效应形成了定性和定量振荡模式,如哺乳动物昼夜节律的出现和变化。这些发现凸显了常规准稳态假设的弊端,并加深了对丰富的时变生物分子活动的机理理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enlightening the blind spot of the Michaelis–Menten rate law: The role of relaxation dynamics in molecular complex formation
The century-long Michaelis–Menten rate law and its modifications in the modeling of biochemical rate processes stand on the assumption that the concentration of the complex of interacting molecules, at each moment, rapidly approaches an equilibrium (quasi-steady state) compared to the pace of molecular concentration changes. Yet, in the case of actively time-varying molecular concentrations with transient or oscillatory dynamics, the deviation of the complex profile from the quasi-steady state becomes relevant. A recent theoretical approach, known as the effective time-delay scheme (ETS), suggests that the delay from the relaxation time of molecular complex formation contributes to the substantial breakdown of the quasi-steady state assumption. Here, we systematically expand this ETS and inquire into the comprehensive roles of relaxation dynamics in complex formation. Through the modeling of rhythmic protein–protein and protein–DNA interactions and the mammalian circadian clock, our analysis reveals the effect of the relaxation dynamics beyond the time delay, which extends to the dampening of changes in the complex concentration with a reduction in the oscillation amplitude compared to the quasi-steady state. Interestingly, the combined effect of the time delay and amplitude reduction shapes both qualitative and quantitative oscillatory patterns such as the emergence and variability of the mammalian circadian rhythms. These findings highlight the downside of the routine assumption of quasi-steady states and enhance the mechanistic understanding of rich time-varying biomolecular processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
4.20
自引率
5.00%
发文量
218
审稿时长
51 days
期刊介绍:
The Journal of Theoretical Biology is the leading forum for theoretical perspectives that give insight into biological processes. It covers a very wide range of topics and is of interest to biologists in many areas of research, including:
• Brain and Neuroscience
• Cancer Growth and Treatment
• Cell Biology
• Developmental Biology
• Ecology
• Evolution
• Immunology,
• Infectious and non-infectious Diseases,
• Mathematical, Computational, Biophysical and Statistical Modeling
• Microbiology, Molecular Biology, and Biochemistry
• Networks and Complex Systems
• Physiology
• Pharmacodynamics
• Animal Behavior and Game Theory
Acceptable papers are those that bear significant importance on the biology per se being presented, and not on the mathematical analysis. Papers that include some data or experimental material bearing on theory will be considered, including those that contain comparative study, statistical data analysis, mathematical proof, computer simulations, experiments, field observations, or even philosophical arguments, which are all methods to support or reject theoretical ideas. However, there should be a concerted effort to make papers intelligible to biologists in the chosen field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


