设计和评估新型 N-取代基二苯基胺衍生物作为管蛋白秋水仙碱结合位点抑制剂。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
基于结构简化和结构融合策略,我们设计了新型 N-取代基二苯基胺衍生物,作为以秋水仙碱结合位点为靶点的小管蛋白抑制剂。所设计的大多数化合物对五种癌细胞株具有中等或强效的抗增殖活性。其中,化合物 4k 对骨肉瘤细胞 MG-63 和 U2OS 具有显著的选择性,IC50 值为 0.08-0.14 μM。进一步的研究证实,4k 可通过靶向秋水仙碱结合位点抑制微管蛋白聚合。同时,化合物 4k 不仅能有效诱导肿瘤细胞周期停滞在 G2/M 期,还能轻微诱导细胞凋亡。这些结果表明,二苯胺衍生物的 N-取代基有望进一步发展成为管蛋白秋水仙碱结合位点抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
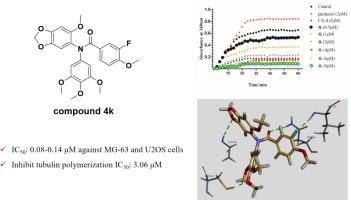
Design and evaluation of novel N-substituent diphenylamine derivatives as tubulin colchicine binding site inhibitors
Novel N-substituent diphenylamine derivatives as tubulin inhibitors targeting colchicine-binding site have been designed based on structural simplification and structural fusing strategy. Most designed compounds exhibited the moderate or potent antiproliferative activities against five cancer cell lines. Among them, compound 4k displayed the significant selectivity for osteosarcoma cells MG-63 and U2OS with the IC50 value of 0.08–0.14 μM. Further investigations verified 4k could inhibit tubulin polymerization by targeting colchicine binding site. Meanwhile, compound 4k not only effectively induced tumor cell cycle arrest at the G2/M phase, but also slightly induced cell apoptosis. These results indicated that N-substituent of diphenylamine derivatives are deserved for further development as tubulin colchicine binding site inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


