优化用于氧还原反应的 PtCu3@Pt/C 催化剂的原子层沉积铂壳厚度
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
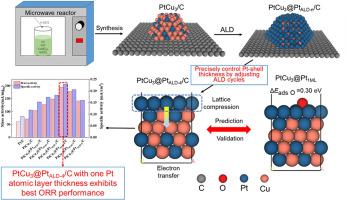
通过湿化学法制备支撑型PtCu3/C和原子层沉积(ALD)技术制备铂壳覆盖型PtCu3纳米颗粒,成功合成了一系列适用于氧还原反应(ORR)的核@壳结构PtCu3@Pt/C催化剂。基于密度泛函理论计算的模型 PtCu3@Pt(111) 表面的氧吸附能表明,适合 ORR 的最佳氧吸附强度出现在铂壳层数较少的 PtCu3@Pt(111) 上。为此,通过改变 1 至 6 次 ALD 周期来精确调节铂壳厚度,结果发现 4 次 ALD 周期可在 PtCu3 纳米粒子表面沉积约一层铂原子。通过材料表征进行的深入研究验证了 PtCu3 合金的形成和铂壳厚度的可调性。在 PtCu3 内核和 Pt 壳之间观察到了应变效应和电子效应,表现为 Pt 壳的晶格压缩和电子从 Pt 带转移到 Cu,这两种效应都会使 Pt 壳的 d 带中心下移,从而削弱对氧物种的吸附。采用旋转盘电极法测试了各种 PtCu3@PtALD-n/C(n = 1-6)催化剂在 ORR 过程中的电催化性能。在所有催化剂中,PtCu3@PtALD-4/C 的质量活性和比活性最高,分别是商用 Pt/C 催化剂的 3.2 倍和 2.6 倍,也远远优于不含铂壳的 PtCu3/C。PtCu3@PtALD-4/C 催化剂的耐久性也优于 PtCu3/C 和 Pt/C 催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Optimization of atomic layer deposited Pt-shell thickness of PtCu3@Pt/C catalyst for oxygen reduction reaction
A series of PtCu3@Pt/C catalysts with core@shell structure applicable to oxygen reduction reaction (ORR) were successfully synthesized by combining wet chemistry method for supported PtCu3/C preparation and atomic layer deposition (ALD) technique for Pt-shell covering PtCu3 nanoparticles. The oxygen adsorption energy on the surface of model PtCu3@Pt(111) based on density functional theory calculation revealed that the optimal oxygen adsorption strength suitable for ORR appears on the PtCu3@Pt(111) having few layers of Pt-shell. For this purpose, the Pt-shell thickness was precisely adjusted by varying the number of ALD cycles between 1 and 6, and four ALD cycles were found to deposit approximately one layer of Pt atoms on the surface of PtCu3 nanoparticles. In-depth investigation through material characterization verified the formation of PtCu3 alloy and the adjustability of Pt-shell thickness. Strain effect and electronic effects were observed between the PtCu3 core and Pt-shell, manifested as lattice compression of the Pt-shell and electron transfer from Pt band to Cu, both of which can downshift d-band center of the Pt-shell thus weakening the adsorption of oxygen species. The electrocatalytic performance of various PtCu3@PtALD-n/C (n = 1–6) catalysts was tested in the ORR process using rotating disk electrode approach. PtCu3@PtALD-4/C exhibited the maximum mass and specific activity among all catalysts, being 3.2 and 2.6 times higher than a commercial Pt/C catalyst, and much better as well than the PtCu3/C without Pt-shell. The durability of the PtCu3@PtALD-4/C catalyst was also superior to that of the PtCu3/C and Pt/C catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


