核心氟化对扇形偶氮苯基超分子的相特性、顺反光异构化和光致发光动力学的影响
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
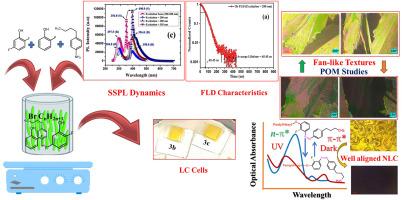
长期以来,刺激响应型、低分子量、动态可调的光物理特征一直是化学家合成液晶(LC)化合物所面临的重要挑战。本文报告了核心氟化对扇形偶氮苯衍生物介形行为的影响。研究人员合成了两个新系列的偶氮苯衍生物(3a-3c、4a-4c),这两个系列的偶氮苯衍生物在末端烷氧基侧链的长度以及偶氮苯分子上氟(-F)基团的取代程度上各不相同,并利用各种分析技术确认了这些化合物的分子结构。LC 化合物 3b 和 3c 的吸收光谱以 350-410 纳米波长附近的 ππ∗ 转变为特征。使用偏振光学显微镜(POM)和差示扫描比色法(DSC)对这些低聚物化合物的自组装进行了研究。LC 化合物 3b 和 3c 的反式-顺式光异构化发生在这些吸收带中。LC 化合物 3b 和 3c 的反式-顺式光异构化过程分别为 4 小时和 24.5 小时,而热顺式-反式异构化率分别为 90s 和 100s,这导致了介相的调整。在 220 nm、230 nm 和 240 nm 处激发时,液相色谱化合物 3b 和 3c 的室温光致发光(RTPL)显示出几条尖锐/微弱的发射光强度带。当在 220 纳米波长下激发时,两种 LC 化合物(3b 和 3c)都显示出尖锐的蓝色发射带和黄色/绿色/橙色带,这些带与弱发射光谱相对应。此外,这两种 LC 化合物的稳态光致发光(SSPL)光谱显示出尖锐的近边缘发射带和宽广的紫色发射峰,具有较高的斯托克偏移和全宽半最大值(FWHM)。化合物 3 b 的荧光寿命衰减(FLD)研究显示,在不同的激发波长下,其平均寿命(τ)穿梭于 17.24 ns 和 103.60 ns 之间。然而,LC 化合物 3c 的 FLD 显示,在不同的激发波长下,τ 在 27.00 ns 和 102.56 ns 之间波动。两种低浓化合物的量子产率(QY)都随着激发波长的增加而降低。研究证明,芳香环一端的烷氧基侧链和核心氟化是改变偶氮苯衍生物 LC 行为的重要工具。因此,合成的偶氮苯衍生物有望用于开发永久性光学存储和显示设备。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Influence of core fluorination on the phase properties of fan-like azobenzene based supramolecules, their cis-trans photoisomerization and photoluminescence dynamics
The stimuli-responsive, low molecular weight, dynamically tunable photophysical features have long been an important objective that challenges chemists in synthesizing liquid crystal (LC) compounds. Herein, the effect of core fluorination on the mesomorphic behavior of fan-like azobenzene derivatives was reported. Two new series of azobenzene derivatives which differ from each other in the length of the terminal alkoxy side chain as well as fluorine (-F) group substitution on the azobenzene moiety were synthesized (3a-3c, 4a-4c) and molecular structures of the compounds were confirmed using various analytical techniques. Absorption spectra of the LC compounds 3b and 3c are characterized by ππ∗ transitions around 350–410 nm. The self-assembly of these LC compounds was investigated using polarized optical microscope (POM) and differential scanning colorimetry (DSC). The trans-to-cis photoisomerization of the LC compounds 3b and 3c occurs in these absorption bands. The trans-to-cis photoisomerization of LC compounds 3b and 3c showed 4 h and 24.5 h whereas, thermal cis-to-trans isomerization rates were found to be 90s and 100s resulting in tuning of mesophases. Room temperature photoluminescence (RTPL) of LC compounds 3b and 3c when excited at 220 nm, 230 nm and 240 nm showed several sharp/weak emission intensity bands. Both the LC compounds (3b, 3c) showed sharp blue emission bands and yellow/green/orange colored bands correspond to weak emission spectra when excited at 220 nm. Further, steady state photoluminescence (SSPL) spectra of these both LC compounds revealed sharp near edge emission bands and broad violet emission peaks with higher Stoke's shift as well as full width half maximum (FWHM). Fluorescence lifetime decay (FLD) studies of compound 3 b unveiled an average lifetime (τ) shuttle between 17.24 ns and 103.60 ns at various excitation wavelengths. However, FLD of LC compound 3c unveiled that the τ fluctuates between 27.00 ns and 102.56 ns at various excitation wavelengths. Quantum yield (QY) decreases for both the LC compounds with an increase in excitation wavelengths. The study proved the importance of the alkoxy side chain at one end of the aromatic ring and core fluorination as a significant tool to modify the LC behavior of azobenzene derivatives. Thus, synthesized azobenzene derivatives are potentially useful for developing permanent optical storage and display devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


