固溶体 Yb(Al,T)B4(T = Mo 或 W)化合物的合成及其某些特性
IF 3.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
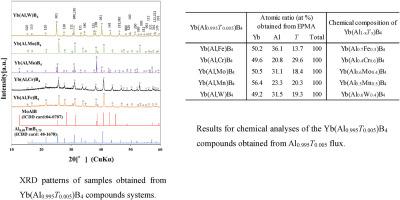
由于最近有关 RAlB4(R = 稀土元素)在磁性、热电和超导材料中特性的报道,它引起了越来越多的关注。作者开发并研究了固溶体 Yb(Al0.995T0.005)B4(T = Cr、Mn 或 Fe)化合物的形成,其中 YbAlB4 的 Al 位置使用 Al flux 方法被 T 原子取代。在这项研究中,通过在 1773 K 下使用混合有 T 金属的铝助熔剂 5 小时,生长出了硼化物 Yb(Al0.995T0.005)B4 (T = Mo 或 W)的晶体。得到的 Yb(Al0.995W0.005)B4 为单相,但在 Yb(Al0.995Mo0.005)B4 中,含有少量 AlMoB 晶体。使用显微维氏硬度计测量了晶体的硬度,并使用 DTA-TG 仪器检测了晶体在空气中的抗氧化性。通过比较 YbAlB4 和 Yb(Al0.995T0.005)B4 的晶格常数和化学成分可知,Yb(Al,T)B4 的晶格常数大于 YbAlB4 晶体的晶格常数。Yb(Al,T)B4以固溶体的形式生长,每种 T 金属的含量约为 40%。Yb(Al0.995Mo0.005)B4 的硬度值为 11.0(±0.7) GPa,Yb(Al0.995W0.005)B4 的硬度值为 15.7(±1.9) GPa。Yb(Al,T)B4 的氧化开始于 971-987 K 之间。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Syntheses and some properties of solid solution Yb(Al,T)B4 (T = Mo or W) compounds
RAlB4 (R = rare earth element) has attracted increasing attention due to recent reports on its properties in magnetic, thermoelectric and superconducting materials. The authors developed and we investigated the formation of the solid solution Yb(Al0.995T0.005)B4 (T = Cr, Mn or Fe) compounds in which the Al position of YbAlB4 is substituted with T atoms using the Al flux method. In this study, crystals of the boride Yb(Al0.995T0.005)B4 (T = Mo or W) were grown by using Al flux mixed with T metal at 1773 K for 5h. Yb(Al0.995W0.005)B4 was obtained as the single phase, however, in the case of Yb(Al0.995Mo0.005)B4, a small amount of AlMoB crystals was contained. The crystals were measured using a micro-Vickers hardness tester, and the oxidation resistance was examined in air using DTA-TG apparatus. Comparing the lattice constants and chemical composition of YbAlB4 and Yb(Al0.995T0.005)B4, it were understood that the lattice constant of Yb(Al,T)B4 were larger than that of YbAlB4 crystals. Yb(Al,T)B4 were grown as a solid solution contained about 40 % of each T metal. The hardness values were 11.0(±0.7) GPa for Yb(Al0.995Mo0.005)B4 and 15.7(±1.9) GPa for Yb(Al0.995W0.005)B4. The oxidation of Yb(Al,T)B4 starts in the range of 971–987 K. The oxidation onset temperature during the heating of Yb(Al,T)B4 is about 100 K higher than YbAlB4.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


