纯化合物参数对二氧化碳+1-己醇二元体系相行为的影响
IF 2.8
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
采用分析-静态方法,通过特殊阀门(由 Ralf Dohrn 教授及其合作者定义的 "AnTVisVarCap")对二氧化碳 (1) + 1- 己醇 (2) 二元体系进行相取样,在六个温度(308.15 K 至 383.15 K)和最高 182.9 K 的压力下测量了新的汽-液-液和汽-液平衡数据,以补充现有数据。这里报告的六个等温线中有四个是首次测量。高压装置的主要部件是一个 60 cm3 的可视池。新的等温线与现有文献进行了比较,并对文献进行了回顾和分析。值得注意的是,在已发表的数据中,只有一个研究小组报告了平衡状态下两相的成分,而我们之前使用的是另一种实验方法。我们使用彭-罗宾逊(PR)和苏韦-雷德里希-邝(SRK)状态方程对新数据和文献数据进行了建模,该模型基于半预测程序,目的是使用一组二元相互作用参数尽可能再现临界曲线的最小值和最大值。讨论了纯组分的临界数据和中心因子对其二元体系相行为的影响。虽然在我们使用的数据库中,纯物质的临界压力和中心因子值相差不大,但模型却能用同一组二元相互作用参数预测出 III 型或 IV 型相态。这种敏感性是我们研究的其他体系所没有的,其原因可能是该体系的醇结构和高度不对称。因此,我们更深入地分析了临界温度和压力以及二氧化碳和 1-己醇的中心因子的影响,并以高于系统 UCEP 温度的一个温度为例进行了说明。本文章由计算机程序翻译,如有差异,请以英文原文为准。
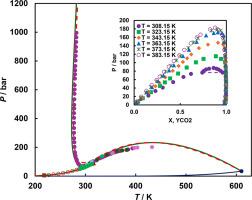
The influence of pure compounds’ parameters on the phase behaviour of carbon dioxide + 1-hexanol binary system
New vapour–liquid–liquid and vapour–liquid equilibrium data to complement the existing ones are measured at six temperatures (308.15 K to 383.15 K) and at pressures up to 182.9 K using an analytic-static method with phases sampling via special valves (“AnTVisVarCap”, as defined by Prof. Ralf Dohrn and co-workers) for the carbon dioxide (1) + 1-hexanol (2) binary system. Four out of the six isotherm reported here are measured for the first time. The main component of the high-pressure setup is a 60 cm3 visual cell.
The new isotherms are compared with the available literature which is also reviewed and analysed. It should be noted that among the data already published, only one other research group reported the compositions of both phases at equilibrium, as we did previously by using another experimental method. The new and literature data were modelled with Peng-Robinson (PR) and Soave-Redlich-Kwong (SRK) equations of state based on a semi-predictive procedure to reproduce as well as possible the minimum and the maximum of the critical curve(s) using one set of binary interaction parameters. The influence of critical data and acentric factors of pure components on the phase behaviour of their binary system is discussed. Although the values of the critical pressures and acentric factors of pure substances are not very different in the database we used, the models predict type III or IV phase behaviour with the same set of binary interaction parameters. This sensitivity, which was not observed for other systems we studied, could be explained by the alcohol structure and high asymmetry of the system. Therefore, we analysed in more depth the influence of the critical temperatures and pressures, as well as the acentric factors of carbon dioxide and 1-hexanol and exemplified for one temperature located above the system UCEP's temperature.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


