以 4-氨基-1,2,4-三唑-3-硫酮的希夫碱抑制金属-β-内酰胺酶为目标:硅对接、分子动力学和药理学评估
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
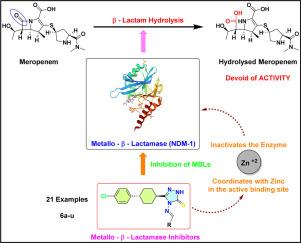
金属-β-内酰胺酶是一种锌依赖性酶,可水解和灭活多种 β-内酰胺类抗生素,包括青霉素类、头孢菌素类和碳青霉烯类,使其成为细菌耐药性的一个重要因素。因此,开发针对金属-β-内酰胺酶的抑制剂对于对抗革兰氏阴性细菌的抗生素耐药性以及恢复这些重要抗菌药物的有效性至关重要。本研究的重点是 21 个由反式-4-氨基-5-(4-(4-氯苯基)环己基)-1,2,4-三唑-3-硫酮衍生的希夫碱类似物(6a-u)的合成、硅对接、分子动力学模拟和生物学评价。对肺炎克雷伯氏菌(PDB:5N0H)和大肠埃希氏菌(PDB:6KXI)的新德里金属-β-内酰胺酶-1 活性位点进行了分子对接研究,以评估配体的硫酸盐形式与锌离子之间的结合相互作用。对接分析表明,合成的类似物稳定地位于 NDM-1 活性位点,与锌离子紧密结合。活性结合位点中的锌离子与三唑环的氮原子和硫代阴离子配位。分子动力学模拟证实了蛋白质-配体复合物的稳定性,表明配体在活性位点内保持最佳定位,周围残基的波动极小。针对表达 NDM-1、A 类(SHV、TEM)或 C 类(CMY)β-内酰胺酶的各种耐碳青霉烯类革兰氏阴性分离菌,评估了这些类似物与美罗培南联用时的体外抗菌活性,浓度分别为 4 mg/L 和 8 mg/L。研究结果表明,美罗培南的抗菌活性有适度的增效作用,与希夫碱类似物同时使用时,药效可提高 2-3 倍。值得注意的是,有两种类似物在这项研究中出现了重大突破。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Targeting metallo-β-lactamase inhibition with Schiff bases of 4-amino-1,2,4-triazole-3-thione: In silico docking, molecular dynamics, and pharmacological assessments
Metallo-β-lactamases are zinc-dependent enzymes that hydrolyze and inactivate a wide range of β-lactam antibiotics, including penicillins, cephalosporins, and carbapenems, making them a significant factor in bacterial resistance. Therefore, developing inhibitors targeting metallo-β-lactamases is crucial for combating antibiotic resistance in Gram-negative bacteria and restoring the effectiveness of these essential antimicrobial agents. This study focuses on the synthesis, in silico docking, molecular dynamics simulations, and biological evaluation of twenty-one Schiff base analogs (6a–u) derived from trans-4-amino-5-(4-(4-chlorophenyl)cyclohexyl)-1,2,4-triazole-3-thione. Molecular docking studies were carried out on the New Delhi metallo-β-lactamase-1 active sites of Klebsiella pneumoniae (PDB: 5N0H) and Escherichia coli (PDB: 6KXI) to evaluate the binding interactions between the thiolate form of ligands and zinc ions. Docking analysis reveals that the synthesized analogs are stably positioned in the NDM-1 active site, aligning closely with zinc ions. The zinc ions in the active binding site coordinate with the nitrogen atoms of the triazole ring and the thiolate anion. Molecular Dynamics Simulations confirmed the stability of the protein-ligand complexes, demonstrating that the ligands maintained optimal positioning within the active site with minimal fluctuations in surrounding residues. The in vitro antibacterial activity of these analogs was evaluated at concentrations of 4 mg/L and 8 mg/L in combination with meropenem against various carbapenem-resistant Gram-negative isolates expressing NDM-1, Class A (SHV, TEM), or Class C (CMY) β-lactamases. The results indicated modest potentiation of meropenem's antibacterial activity, with a 2–3 fold increase in efficacy when used alongside the Schiff base analogs. Notably, two analogs emerged as significant hits in this study.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


