黄芩苷纳米纤维膜:关于制备、表征和抗菌药理活性的综合研究
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
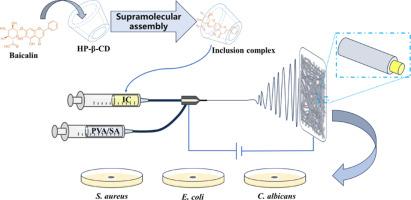
黄芩苷是一种天然活性成分,因其药用特性和广泛的药理活性而闻名,包括抗菌、抗氧化、抗病毒和抗癌作用。然而,水溶性差阻碍了它的应用。在本研究中,我们旨在通过超分子组装将黄芩苷嵌入羟丙基-β-环糊精的疏水空腔中,形成包合物,从而提高黄芩苷的水溶性。随后,利用同轴静电纺丝技术将包合物加工成适用于皮肤和伤口的纳米纤维膜。利用高效液相色谱(HPLC)、X 射线衍射(XRD)、傅立叶变换红外光谱(FT-IR)、热重分析(TGA)、扫描电子显微镜(SEM)和透射电子显微镜(TEM)对包合物和纳米纤维膜进行了分析和表征。X射线衍射、傅立叶变换红外和热重分析的综合结果证实,黄芩苷成功地与环糊精形成了包合物,使水溶性从 0.0170 毫克/毫升提高到 8.9600 毫克/毫升,提高了 527 倍。此外,与原生黄芩苷相比,包合物对大肠杆菌、金黄色葡萄球菌和白僵菌具有更强的抗菌活性和更高的自由基清除率。通过同轴电纺将包合物转化为纳米纤维膜后,它们的性质保持不变,继续表现出优异的抗菌和抗氧化活性。在体外释放试验中,近 90% 的药物(黄芩苷)在一段时间内从纳米纤维膜中释放出来,表明其具有持续释放性。此外,通过分子对接模拟,阐明了黄芩苷与环糊精的分子对接机理,为计算研究中包合物的合成奠定了理论基础。本研究成功制备了含有生物黄芩苷的水溶性抗菌纳米纤维膜,它在利用植物提取的生物活性化合物开发伤口敷料和给药系统方面具有潜在的临床应用价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Baicalin nanofiber membranes: A comprehensive study on preparation, characterization, and antimicrobial pharmacological activities
Baicalin is a natural active ingredient known for its medicinal properties and a broad spectrum of pharmacological activities, including antimicrobial, antioxidant, antiviral, and anticancer effects. However, its application is hindered by its poor water-solubility. In this study, we aimed to enhance the water solubility of baicalin by embedding it within the hydrophobic cavity of hydroxypropyl-β-cyclodextrin through supramolecular assembly to form inclusion complexes. The inclusion complexes were subsequently processed into nanofiber membranes suitable for application on skin and wounds using coaxial electrostatic spinning. The inclusion complexes and nanofiber membranes were analyzed and characterized using high-performance liquid chromatography (HPLC), X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). The combined results from XRD, FT-IR, and TGA confirmed that baicalin successfully formed inclusion complexes with cyclodextrin, resulting in an increase in water solubility from 0.0170 mg/mL to 8.9600 mg/mL, representing a 527-fold enhancement. Additionally, the inclusion complexes demonstrated superior antimicrobial activity against E. coli, S. aureus and C. albicans, as well as higher free radical scavenging rates compared to native baicalin. After the inclusion complexes were transformed into nanofiber membranes via coaxial electrospinning, their properties remained unchanged, and they continued to exhibit excellent antimicrobial and antioxidant activities. In the in vitro release assay, nearly 90 % of the drug (baicalin) was released from the nanofiber membranes over a period of time, indicating sustained release. Furthermore, the molecular docking mechanism of baicalin and cyclodextrin was elucidated through molecular docking simulations, establishing a theoretical foundation for the synthesis of inclusion complexes in the computational studies. This study reports the successful fabrication of water-soluble, antimicrobial nanofiber membranes containing bioavailable baicalin, which have potential clinical applications in the development of wound dressings and drug delivery systems utilizing plant-extracted bioactive compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


