从废弃生物质中获得碳点的宽光致发光斯托克斯位移
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
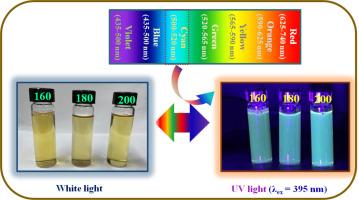
由于碳量子点(CQDs)具有巨大的物理化学和量子约束特性,它们在能量转换和能量存储领域展现出令人兴奋的重要前景。然而,由于其光致发光量子产率(PLQY)低和/或斯托克斯位移短,限制了其在光伏(PV)领域的应用。为此,我们采用了一种新颖且具有成本效益的方法,利用废弃生物质合成 CQDs。此外,还进行了一项综合研究,以探索 CQDs 作为光子降频器/降频器在光伏中的适用性。在 160、180 和 200 ℃ 的合成温度下,CQDs 的平均尺寸分别为 5.1、4.4 和 3.9 nm。EDS 分析表明,所有 CQDs 中都含有 C、O、Mg、Na、Cl 和 K。随着合成温度的升高,PL 发射峰位置发生蓝移。随着合成温度的升高,斯托克斯位移减小。在 160 ℃ 下合成的 CQDs 在激发波长为 300 nm 时的斯托克斯位移最大。温度从 160 ℃ 升至 180 ℃ 和 200 ℃ 时,PLQY 分别增加了 3.5 倍和 7.2 倍。研究结果表明,通过选择合成参数可以丰富 CQDs 的适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Attaining a wide photoluminescence Stokes-shift of carbon dots obtained from waste biomass
Owing to the tremendous physicochemical and quantum confinement characteristics of carbon quantum dots (CQDs), they have revealed exciting and essential visions in the field of energy conversion and energy storage. However, due to their low photoluminescence quantum yield (PLQY) and/or short Stokes-shift, limits their application in photovoltaic (PV). In this regard, a novel and cost-effective method was used to synthesize the CQDs using waste biomass. Furthermore, a comprehensive study was made to explore the applicability of the CQDs as photon downconverters/downshifters in PVs. The average size of the CQDs are 5.1, 4.4, and 3.9 nm for the synthesis temperature of 160, 180, and 200 ℃, respectively. The EDS analysis revealed that C, O, Mg, Na, Cl, and K are present in all the CQDs. The PL emission peak position is blue-shifted with the rise in the synthesis temperature. The Stokes-shift is reduced with a rise in the synthesis temperature. The highest Stokes-shift was obtained at excitation wavelength of 300 nm for CQDs synthesized at 160 ℃. The PLQY increased by 3.5 and 7.2-folds for the rise in the temperature from 160 to 180 and 200 ℃, respectively. The investigation revealed that the applicability of CQDs can be enriched via the selection of the synthesis parameters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


