基于阿司他丁的有机锡羧酸盐的合成、晶体结构和抗癌活性
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用元素分析、红外光谱、核磁共振光谱和热重分析技术合成并表征了六种基于阿司他丁配体(HL)的有机锡羧酸盐。通过单晶 X 射线晶体学研究证实了 a-f 复合物的分子结构。阿司他丁显示出采用各种配位模式的趋势,从而在有机锡羧酸盐中形成了不同的分子构型。Hirshfeld表面分析表明,六种复合物的Hirshfeld表面呈现出类似的不同接触,其中H--H/C/O相互接触占Hirshfeld总表面的91%以上。针对三种人类癌细胞系(NCI-H460、HepG2 和 MCF7)的 CCK8 试验评估了所有复合物的体外抗癌活性。通过引入丁基锡基,阿司他丁的抗癌活性得到了有效提高,并在体外表现出卓越的抗癌活性,明显优于顺铂。通过紫外可见吸收光谱法和荧光竞争法研究了复合物 d 的 DNA 结合情况。本文章由计算机程序翻译,如有差异,请以英文原文为准。
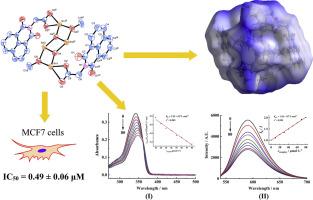
Syntheses, crystal structures, and anticancer activities of organotin carboxylates based on Alrestatin
Six organotin carboxylates based on the Alrestatin ligand (HL) were synthesized and characterized using elemental analysis, IR spectroscopy, NMR spectroscopy, and TGA techniques. The molecular structure of complexes a-f was confirmed through single-crystal X-ray crystallography. Alrestatin demonstrated a tendency to adopt various coordination modes, leading to the formation of diverse molecular configurations in the organotin carboxylates. Hirshfeld surface analysis indicated that the six complexes exhibited similar contributions of different contacts to the Hirshfeld surfaces, with reciprocal H···H/C/O contacts dominating over 91 % of the total Hirshfeld surface. The in vitro anticancer activities of all the complexes were evaluated by a CCK8 assay against three human cancer cell lines (NCI-H460, HepG2, and MCF7). The anticancer activity of Alrestatin was effectively increased by introducing the butyl tin group, and exhibited excellent anticancer activity in vitro, significantly superior to cisplatin. The DNA binding of complex d was studied by UV–visible absorption spectrometry and fluorescence competitive assays.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


