可见光促进的无催化剂异烟肼氮杂环丁烷合成:体外抗氧化活性、分子对接、ADME 和毒性预测
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
生物活性分子的绿色合成对环境可持续性、资源效率、减少环境影响和遵守法规至关重要。它确保了更安全的工作环境,并促进了可持续化学实践的创新。在此背景下,我们介绍了一种电子供体-受体(EDA)介导的可见光促进、无催化剂(VLCF)的可扩展异烟肼氮杂环丁烷合成方法。该合成包括药物沙利肼及其类似物,随后对它们的抗氧化活性进行了评估。与沙利肼和抗坏血酸相比,异烟肼氮杂环丁烷 3e 的活性更强,在过氧化氢抗氧化试验中的 IC50 值为 0.078 毫克/毫升。具体来说,3e 的抗氧化性(79.02%)高于异烟肼偶氮甲烷 3b(75.82%)、异烟肼偶氮甲烷 3d(62.2%)、异烟肼偶氮甲烷 3c(59.86%)和异烟肼偶氮甲烷 3a(54.62%)。在超氧化物歧化酶抗氧化试验中,3e 也被确定为活性最高的化合物,其 SOD 活性水平为 650 U/mg 蛋白,超过了其他 SOD 活性水平分别为 330、560、350 和 420 U/mg 蛋白的化合物(3a-d)。与辣根过氧化物酶(1W4Y)的分子对接显示,化合物 3e 的结合能最好(-6.561 kcal/mol),与 Arg38 形成了关键的氢键(Asn135、Pro139)和π阳离子相互作用。这些相互作用表明 3e 可有效抑制过氧化氢催化作用。对合成化合物的理化性质、药代动力学和毒理学进行的硅学评估表明,这些化合物表现出良好的 ADMET 特性,没有发现任何毒理学问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
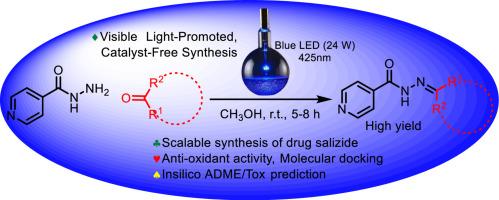
Visible light-promoted, catalyst-free synthesis of isoniazid azomethines: In vitro antioxidant activity, molecular docking, ADME and toxicity prediction
Green synthesis of biologically active molecules is essential for environmental sustainability, resource efficiency, reduced environmental impact, and compliance with regulations. It ensures a safer working environment, and fosters innovation in sustainable chemistry practices. In this context, we introduce an electron donor-acceptor (EDA)-mediated visible light-promoted, catalyst-free (VLCF) scalable synthesis of isoniazid azomethines. This synthesis encompasses the drug salizide and its analogues, which were subsequently evaluated for their antioxidant activities. Isoniazid azomethines 3e demonstrated superior activity compared to salizide and ascorbic acid, with an IC50 of 0.078 mg/mL in the hydrogen peroxide antioxidant assay. Specifically, 3e exhibited greater antioxidant properties (79.02 %) than isoniazid azomethine 3b (75.82 %), isoniazid azomethine 3d (62.2 %), isoniazid azomethine 3c (59.86 %), and isoniazid azomethine 3a (54.62 %). In the superoxide dismutase antioxidant assay, 3e was also identified as the most active, with a SOD activity level of 650 U/mg of protein, surpassing other compounds (3a-d) with SOD activity levels of 330, 560, 350, and 420 U/mg of protein, respectively. Molecular docking against horseradish peroxidase (1W4Y) showed compound 3e with the best binding energy (-6.561 kcal/mol), forming key hydrogen bonds (Asn135, Pro139) and a π-cation interaction with Arg38. These interactions suggest 3e may effectively inhibit hydrogen peroxide catalysis. The in silico assessment of the physicochemical properties, pharmacokinetics, and toxicology of synthesized compounds suggests that these compounds exhibit promising ADMET characteristics, with no identified toxicological concerns.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


