C3N 和 H2 之间的非共价相互作用
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
非共价相互作用在许多领域,尤其是物理储氢领域发挥着重要作用。本文通过 DFT 计算研究了 C3N 的储氢特性。结果表明,H2 与 C3N 形成物理吸附。电子结构分析表明,H2 和 C3N 之间的共价作用和静电作用都相当微弱,而 IRI 分析表明它们之间的相互作用属于非价作用,基于 SAPT 的进一步能量分解表明,TCR 和 TNR 两种构型的相互作用能量来源不同。对于 TCR,感应能是主要的贡献者,而对于 TNR,静电相互作用则占主导地位。我们的综合研究不仅加深了我们对 H2 和 C3N 之间错综复杂的相互作用的理解,还为提高物理储氢系统的吸附强度提供了宝贵的指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
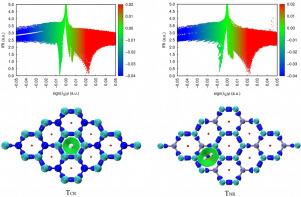
The non-covalent interaction between C3N and H2
Non-covalent interactions play an important role in numerous fields, particularly in physical hydrogen storage. The hydrogen storage properties of C3N are investigated by DFT calculations. The results indicated that H2 and C3N form physical adsorption. The electronic structure analysis demonstrates that both the covalent and electrostatic interactions between H2 and C3N are rather weak, while IRI analysis reveals that their interactions belong to non-valent interactions, and the further energy decomposition based on SAPT suggests that the sources of interaction energies differ for the two configurations TCR and TNR. For TCR, the induction energy is the primary contributor, for TNR, the electrostatic interaction dominates. Our comprehensive study not only enhances our understanding of the intricate interactions between H2 and C3N but also serves as a valuable guide for enhancing the adsorption strength in physical hydrogen storage systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


