掺杂硼嗪的纳米石墨烯(CBNG)薄片是一种前景广阔的二氧化氮气体传感器:理论家的方法
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
纳米石墨烯(NGs)是石墨烯的一个独特部分,其中的悬键被氢原子(C42H18)饱和。这种尺寸限制带来了独特的性能和潜在的应用。本文采用密度泛函理论(DFT)研究了掺杂硼嗪(B2C2N2)环的纳米石墨烯(CBNG)模型在检测二氧化氮气体时的反应性和电子灵敏度。在 CBNG/NO2 复合物中,NO2 分子的一个氧原子位于距离 B2C2N2 环中心 2.93 英里处,吸附能为 -6.2 kcal/mol。二氧化氮在 CBNG 上的吸附能明显大于其他气体分子,介于强物理吸附和弱化学吸附的合适范围之间。吸附 NO2 后,CBNG 片材的带隙达到 62.4%。本研究成果可用于工业领域,特别是合成有效的二氧化氮气体传感器。本文章由计算机程序翻译,如有差异,请以英文原文为准。
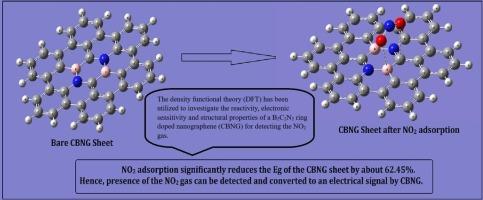
Carborazine doped nanographene (CBNG) sheet as a promising NO2 gas sensor: A theoretician’s approach
Nanographenes (NGs) are a distinct section of graphene in which the dangling bonds are saturated with hydrogen atoms (C42H18). This confinement in size results in unique properties and potential applications. The density functional theory (DFT) is used to investigate the reactivity and electronic sensitivity of a model carborazine (B2C2N2) ring doped nanographene (CBNG) for detecting NO2 gas. In CBNG/NO2 complex, one oxygen atom of the NO2 molecule is positioned at a distance of 2.93Ả from the center of the B2C2N2 ring, with an adsorption energy −6.2 kcal/mol. The adsorption energy of NO2 on CBNG is apparently larger than those of other gas molecules, which fall between the suitable range of strong physical adsorption and weak chemical adsorption. Upon NO2 adsorption, the band gap of CBNG sheet reaches 62.4 %. The outcome of the present study may be exploited in the industry, particularly in the synthesis of effective NO2 gas sensors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


