水溶液中胶束形成和环糊精-表面活性剂包合物形成的平行(竞争)反应:吉布斯-杜恒方程的一个结果--质量作用定律的不变性和确定包合物平衡常数的可能性
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
根据吉布斯-杜恒方程,胶束形成的标准吉布斯自由能与单组分表面活性剂和环糊精(CD)在水溶液中形成包合物的标准吉布斯自由能之间是独立的。实验数据表明,表面活性剂的临界胶束浓度会随着 CD 的存在而增加。在恒温条件下,胶束形成的标准吉布斯自由能保持不变,而与包合物形成等平行竞争过程无关。这表明临界胶束浓度在相应的浓度范围内保持不变。因此,可以通过改变存在和不存在 CD 时的临界胶束浓度来确定表面活性剂-CD 包合物形成的平衡常数。此外,还介绍了一种确定包合复合物平衡常数的图形方法。还提出了一种分析方法,用于分析与 CD 形成的包合物对形成二元混合胶束假相的影响。假设两种表面活性剂二元混合物形成包合物的平衡常数存在不同值。在这种情况下,初始表面活性剂混合物的初始摩尔分数会发生变化,在确定存在 CD 的二元混合胶束的参数时,应用常规溶液理论规程时应考虑到这一点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
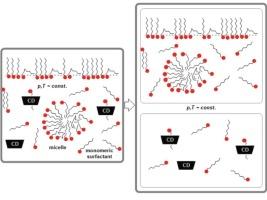
Parallel (competitive) reactions of micelle formation and cyclodextrin–surfactant inclusion complex formation in aqueous solution: A consequence of the Gibbs–Duhem equation—invariance of the mass action law and the possibility of determining the equilibrium constant of the inclusion complex
The Gibbs–Duhem equation results in the independence between the standard Gibbs free energy of micelle formation and the standard Gibbs free energy of monocomponent surfactant’s and cyclodextrin’s (CD’s) inclusion complex formation in aqueous solution. The experimental data show that the surfactant’s critical micellar concentration increases in the presence of CD. At a constant temperature, the standard Gibbs free energy of micelle formation remains constant, irrespective of the presence of parallel competitive processes such as inclusion complex formation. This indicates that the critical micellar concentration remains unchanged at the corresponding concentration scale. As a result, the equilibrium constant for surfactant–CD inclusion complex formation can be determined by changing the critical micellar concentration in the presence and absence of CD. A graphical method for determining the equilibrium constant of the inclusion complex is also presented. An analytical approach for analyzing the influence of the formation of the inclusion complex with CD on the formation of the binary mixed micellar pseudophase is presented. Suppose that different values exist for the equilibrium constants of the formation of the inclusion complex for two surfactants from their binary mixture. In this case, there is a change in the initial mole fraction from the initial mixture of surfactants, which should be considered when applying the regular solution theory protocol when determining the parameters of a binary mixed micelle in the presence of CD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


