由 Zn(II) 和 5-aminotetrazole 组装而成的三维富氮耐热超分子 MOF 具有优异的能量性能
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
耐热炸药对于要求高热稳定性的特殊应用至关重要,在民用和军用领域,尤其是在极端环境下,都是必不可少的。我们的研究重点是利用 5-aminotetrazole (HATZ) 配体和 Zn(II) 离子,合成三维富氮超分子高能金属有机框架(MOF)Zn(ATZ)2 (1)。结构分析表明,化合物 1 采用正交 CmCm 空间群,具有坚固的二维网络,二维层之间具有很强的 π-π 填料相互作用,热稳定性极高,温度可达 322 ℃,机械敏感性极低。经计算,化合物 1 的标准摩尔形成焓(ΔfHo)和引爆热(ΔHdet)分别为 7.08 kJ/g 和 7.34 kJ/g。值得注意的是,化合物 1 的ΔfHo 大大超过了 RDX(0.32 kJ/g)、HNS(0.17 kJ/g)、TNT(-0.295 kJ/g)和 TATB(-0.54 kJ/g)等传统耐热炸药,也高于大多数已报道的高能 MOF 材料。同样,其 ΔHdet 值也超过了 TNT(4.144 kJ/g)、HMX(5.525 kJ/g)和 RDX(5.71 kJ/g)等普通炸药以及大多数已报道的高能 MOF 材料。化合物 1 还具有令人印象深刻的引爆特性,其速度 (D) 为 7.22 km s-1,压力 (P) 为 21.95 GPa,优于许多其他高能 MOF 材料。这些优异的高能特性使化合物 1 成为耐热炸药的有力候选材料,展示了其未来在苛刻环境中的应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
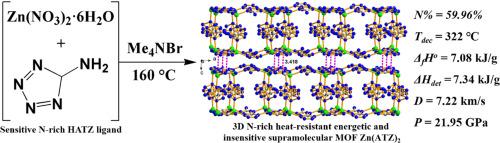
A 3D nitrogen-rich heat-resistant supramolecular MOF with superior energetic performances assembled from Zn(II) and 5-aminotetrazole
Heat-resistant explosives are pivotal for specialized applications demanding high thermal stability, essential in both civil and military sectors, especially in extreme environments. Our study focuses on the synthesis of a 3D nitrogen-rich supramolecular energetic metal-organic framework (MOF), Zn(ATZ)2 (1), using the 5-aminotetrazole (HATZ) ligand and Zn(II) ion. Structural analysis indicates that compound 1 adopts the orthorhombic CmCm space group and possesses a robust 2D network, characterized by strong π-π packing interactions between 2D layers, offering exceptional thermal stability up to 322 °C with minimal mechanical sensitivity. The standard molar enthalpy of formation (ΔfHo) and heat of detonation (ΔHdet) for compound 1 are calculated to be 7.08 kJ/g and 7.34 kJ/g, respectively. Remarkably, the ΔfHo of compound 1 significantly exceeds those of traditional heat-resistant explosives like RDX (0.32 kJ/g), HNS (0.17 kJ/g), TNT (–0.295 kJ/g), and TATB (–0.54 kJ/g), and is also higher than those of most reported energetic MOF materials. Similarly, its ΔHdet value surpasses those of common explosives such as TNT (4.144 kJ/g), HMX (5.525 kJ/g) and RDX (5.71 kJ/g), as well as the majority of reported energetic MOFs. Compound 1 also demonstrates impressive detonation properties with a velocity (D) of 7.22 km s-1 and a pressure (P) of 21.95 GPa, outperforming many other energetic MOF materials. These superior energetic characteristics position compound 1 as a strong candidate for heat-resistant explosives, showcasing its potential for future applications in demanding environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


