新型 vatalanib 类似物:抗癌活性的设计、合成、硅学研究和生物学评价
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
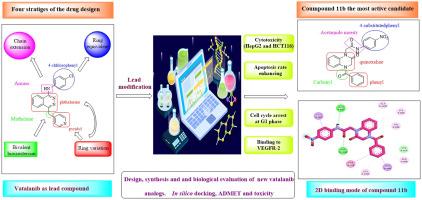
为了改善伐他拉尼的药代动力学和药效学特性,我们采用了四种药物设计策略来设计和合成新的喹喔啉类伐他拉尼类似物。以vatalanib为阳性对照,研究了合成化合物对两种肿瘤细胞系(HepG2和HCT-116)的抗增殖活性。新候选化合物对两种受测癌细胞株显示出良好的抗癌活性,IC50 值范围为 6.04 ± 0.19 至 100.15 ± 3.26 µM,尤其是化合物 11b 和 11c,其效力高于 vatalanib。对 HepG2 和 HCT116 的药效最强的是 4-苯甲酰基喹喔啉衍生物 11b(IC50 = 6.04 ± 0.19 µM 和 15.58 ± 0.58 µM),而伐他拉尼的 IC50 分别为 25.27 ± 0.84 µM 和 43.91 ± 1.64 µM。研究发现,11b 对 HepG2 和 HCT116 的选择性指数分别约为 14 和 5。而 vatalanib 对 HepG2 和 HCT116 的选择性指数分别约为 5 和 3。研究人员在 HepG-2 细胞中进一步研究了最有前景的化合物 11b 的凋亡效应、细胞周期停滞和体外 VEGFR-2 抑制活性,并将其作为细胞死亡的主要机制。研究发现,11b 能诱导细胞凋亡并使细胞周期停滞在 G1 期,此外,与 vatalanib(IC50 为 0.265 ± 0.009 µM)相比,化合物 11b 能有效抑制 VEGFR-2,IC50 为 0.918 ± 0.033 µM。对设计的化合物进行了深入的计算研究,包括分子对接、理化性质、药代动力学分析、ADMET 研究和毒性预测,以评估潜在的候选药物。对接研究结果与生物测试结果一致,此外,硅学 ADMET 研究显示,新候选药物的药代动力学特性优于伐他拉尼。因此,目前的工作表明,4-苯甲酰基喹喔啉-1-基乙酰胺衍生物,尤其是 11b ,可以作为开发新的有效抗癌药物的先导分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
New vatalanib analogs: Design, synthesis, in silico study and biological evaluation for anticancer activity
In our attempts to improve pharmacokinetics and pharmacodynamics characters of vatalanib, four strategies of the drug design were applied to design and synthesis new quinoxaline based vatalanib analogs. Antiproliferative activities of the synthesized compounds were investigated against two tumor cell lines (HepG2 and HCT‐116) using vatalanib as a positive control. The new candidates showed promising anticancer activity against two tested cancer cell lines with IC50 values ranging from 6.04 ± 0.19 to 100.15 ± 3.26 µM, in particular compounds 11b and 11c which were more potent than vatalanib. The most potent was the 4-benzoylquinoxaline derivative 11b (IC50 = 6.04 ± 0.19 µM and 15.58 ± 0.58 µM) compared to vatalanib (IC50 of 25.27 ± 0.84 µM and 43.91 ± 1.64 µM) against HepG2 and HCT116, respectively. The selectivity indices of 11b were found to be approximately 14 and 5 towards HepG2 and HCT116, respectively. While vatalanib showed selectivity indices of about 5 and 3 towards HepG2 and HCT116, respectively. The most promising compound 11b was further investigated in HepG‐2 cells for its apoptotic effect, cell cycle arrest and in vitro VEGFR-2 inhibitory activity as the main mechanisms of cell death. It was found that 11b can induce apoptosis and arrest the cell cycle at the at G1 phase in addition to, compound 11b showed effective inhibition of VEGFR-2 with IC50 of 0.918 ± 0.033 µM compared to vatalanib (IC50 of 0.265 ± 0.009 µM). Deep computational studies were created for the designed compounds including molecular docking, physicochemical properties, profiling pharmacokinetics, ADMET studies, and toxicity predictions to evaluate the prospective drug candidates. The results of the docking study were consistent with biological testing, furthermore, in silico ADMET study revealed the superior pharmacokinetics characters of the new candidates over vatalanib. So, the current work suggests that the 4-benzoylquinoxaline-1-yl-acetamide derivatives, especially 11b, can serve as lead molecules for development of new effective anticancer drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


