计算筛选和研究配体对 TM 单原子氢进化反应催化剂的影响
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
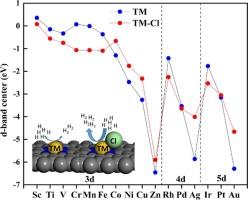
在此,我们研究了过渡金属单原子催化剂的稳定性、电子特性和 HER 催化性能,包括氯配体存在和不存在的情况。研究结果表明,除 Au、Au-Cl、Ag、Ag-Cl 外,所有催化剂都具有热力学稳定性。我们发现 3d 过渡金属比 4d 和 5d 系列的金属更稳定。Ag-Cl、Sc、Ag、Fe-Cl 和 Cr-Cl 的电化学稳定性最差。我们发现 TM-Cl 的费米能与形成能之间存在负的皮尔逊相关性,这与裸 TM 催化剂的趋势相反。早期 3d TM 的氯键体系具有更高的 HER 活性,这表明 d 带中心的下移促进了活性的提高。我们还观察到 TM 和 TM-Cl 体系的 d 波段中心呈周期性变化趋势。在我们的研究系列中,Mn-Cl、Cr-Cl、Ti-Cl、Fe-Cl、V-Cl 和 Zn 成为最优秀的催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Computational screening and investigation of ligand effect on TM single atom catalyst for hydrogen evolution reaction
Here, we studied the stability, electronic characteristics, and HER catalytic performance of transition metal single atom catalysts, both with and without the presence of chlorine ligand. The findings indicate that all catalysts, except for Au, Au-Cl, Ag, Ag-Cl, are thermodynamically stable. We found 3d transition metals are more stable than those in the 4d and 5d series. Ag-Cl, Sc, Ag, Fe-Cl, and Cr-Cl have the poorest electrochemical stability. We found a negative Pearson correlation between Fermi energy and formation energy for TM-Cl, which is opposite to the trend observed for bare TM catalysts. The higher HER activity of chlorine bonded system for the early 3d TM suggests that the downshifting of the d-band center facilitates the activity. We also observed a periodic trend in the d-band center for both TM and TM-Cl systems. Mn-Cl, Cr-Cl, Ti-Cl, Fe-Cl, V-Cl and Zn emerged as the superior catalysts in our study series.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


