使用没食子酸作为辉钼矿抑制剂提高 Mo-Pb 浮选分离效果:浮选行为和机理研究
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
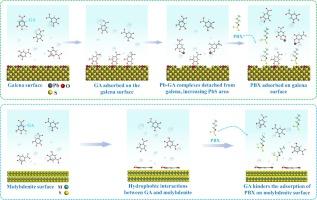
在钼铅大块精矿的浮选分离和含铅辉钼矿精矿的提纯过程中,通常会添加抑制剂来抑制方铅矿或辉钼矿,以获得高质量的浮选产品。以往的研究主要集中在方铅矿的抑制方面。在本研究中,没食子酸(GA)被用作钼铅浮选分离过程中的辉钼矿抑制剂。单矿物浮选实验表明,pH 值对方铅矿和辉钼矿的浮选有显著影响,尤其是在 pH 值为 10 时,GA 的回收率差异最大。混合矿物浮选实验显示了 GA 对辉钼矿的选择性抑制作用。在 GA 的最佳用量下,钼的回收率降低了 76.62 个百分点,而铅的回收率则未受影响。采用接触角、红外光谱、Zeta 电位、电感耦合等离子体光发射光谱(ICP-OES)、X 射线光电子能谱(XPS)分析、第一原理计算分析和界面相互作用自由能计算等方法研究了 GA 的抑制和分离机理。结果表明,GA 处理能明显降低辉钼矿表面的疏水性,但会轻微增强方铅矿表面的疏水性。在 pH 值为 10 时,GA 溶液中的主要物种是 HGA3-。它以 Pb-GA 复合物的形式吸附在方铅矿表面的 PbO 物种上,在高吸附容量时往往会从表面脱离,导致方铅矿不受 GA 的影响。不同的是,GA 通过疏水吸引力吸附在辉钼矿表面,使其具有亲水性。此外,用黄原酸丁酯钾(PBX)处理后,GA 处理过的方铅矿的疏水性显著增强,而 GA 处理过的辉钼矿的疏水性却没有恢复。这是因为PBX可以稳定地吸附在GA处理过的方铅矿上,但它在GA处理过的辉钼矿上的吸附却受到了预先吸附的GA的阻碍。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancement of Mo-Pb flotation separation using gallic acid as a molybdenite depressant: Investigating flotation behavior and mechanism
In the flotation separation of Mo-Pb bulk concentrate and the purification process of Pb-containing molybdenite concentrate, depressants are typically added to depress galena or molybdenite, aiming to obtain high-quality flotation products. Previous studies have focused on the depression of galena. In this study, gallic acid (GA) was utilized as a molybdenite depressant during Mo-Pb flotation separation. Single mineral flotation experiments showed that pH notably influenced galena and molybdenite flotation with GA, particularly at pH 10, displaying the most substantial recovery difference. Mixed mineral flotation experiments revealed GA’s selective depression on molybdenite. At the optimal dosage of GA, the Mo recovery was reduced by 76.62 percentage points, while the Pb recovery remained unaffected. The depression and separation mechanisms of GA were investigated using contact angle, infrared spectroscopy, Zeta potential, inductively coupled plasma optical emission spectrometer (ICP-OES), X-ray photoelectron spectroscopy (XPS) analysis, first-principles calculations analysis, and interface interaction free energy calculations. The results indicate that GA treatment significantly reduces the hydrophobicity of molybdenite surfaces; however, it slightly enhances the hydrophobicity of galena surfaces. At pH 10, the primary species in GA solution is HGA3-. It adsorbs on PbO species of the galena surface as Pb-GA complexes and tends to detach from the surface at high adsorption capacity, resulting in galena not being depressed by GA. Differently, GA adsorbs on the molybdenite surface through hydrophobic attraction force, rendering it hydrophilic. Additionally, after treatment with potassium butyl xanthate (PBX), the hydrophobicity of GA-treated galena is significantly enhanced, while the hydrophobicity of GA-treated molybdenite is not restored. This is because PBX can adsorb stably on GA-treated galena, but its adsorption on GA-treated molybdenite is hindered by the pre-adsorbed GA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


