N-苯乙基-亚氨基二乙酸酯(2-)螯合物的镍(II)二元和三元配合物的超分子结构、Hirshfeld 表面分析、光谱、计算和光伏特性
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
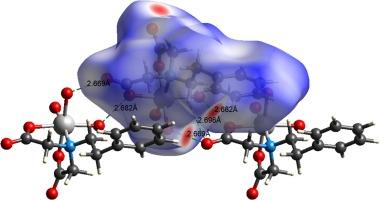
羟基碳酸镍(II)和 N-苯乙基-亚氨基二乙酸(H2pheida)螯合配体在水介质中与化合物(2)[Ni(pheida)(Him)3]-4H2O 和不含咪唑化合物(1)[Ni(pheida)(H2O)3]发生化学反应,从溶液中分离出蓝绿色的镍(II)二元和/或三元化合物。二元化合物[Ni(pheida)(H2O)3](1)的晶体形态不同于相关的四水三元复合物[Ni(pheida)(Him)3]-4H2O(2),这可归因于咪唑配体中存在较强的 N-(Him)间隔。本文报告的化合物已通过元素分析、傅立叶变换红外光谱、紫外可见光、热重分析和 X 射线晶体学等方法进行了表征。此外,还利用 Hirshfeld 表面分析法研究了独特的分子间作用力,特别是 H 键相互作用、二维指纹图谱和超分子结构。镍(II)二元和三元配合物(1)和(2)显示出围绕镍(II)离子的 1 + 2 + 2 + 1 型扭曲八面体几何结构,该几何结构由 3d8 电子构型施加,分别具有退化的全占和半占 t2g6 和 eg2 轨道。与络合物(1)相比,络合物(2)的约翰-泰勒畸变更高。在复合物(1)和(2)中,螯合物的 IDA 臂都采用了 fac-NO2 构象。此外,通过紫外可见吸收数据,利用陶氏方程(αhν = A(hν-Eg)r,其中 r = ½ 表示间接电子转变,2 表示直接电子转变)估算了配体及其相应 Ni(II) 复合物作为半导体的光伏特性。计算得出的复合物 (1) 的间接和直接电子转变带隙(Eg)分别为 2.0 和 3.07 eV。同样,复合物 (2) 的测量值分别为 2.55 和 4.39 eV。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Supramolecular architectures, Hirshfeld surfaces analysis, spectroscopic, computational and photovoltaic properties of nickel(II) binary and ternary complexes of N-phenethyl-iminodiacetate (2-) chelate
The bluish-green color nickel(II) binary and/or ternary compounds have been isolated from the solution of stoichiometric reaction between Ni(II) hydroxy-carbonate and N-phenethyl-iminodiacetic acid (H2pheida) chelating ligand in aqueous media with compound (2); [Ni(pheida)(Him)3]·4H2O and without imidazole compound (1); [Ni(pheida)(H2O)3]. The crystal pattern of binary compound [Ni(pheida)(H2O)3] (1) differs from the related tetra-hydrated ternary complex of [Ni(pheida)(Him)3]·4H2O (2) can be attributed to the presence of stronger N-(Him) spacer of imidazole ligand. The compounds reported herein, have been characterized by means of elemental analysis, FTIR, UV–vis, TGA and X-ray crystallography. Moreover, the distinctive inter-molecular forces particularly H-bonding interactions, 2D fingerprint plots and supramolecular architecture have been studied using Hirshfeld surface analysis. The nickel(II) binary and ternary complexes (1) and (2) show distorted octahedral geometry around the Ni(II) ion of the type 1 + 2 + 2 + 1 imposed by 3d8 electronic configuration with degenerate fully and half occupied t2g6 and eg2 orbitals respectively. The higher John-Teller distortion has been observed for complex (2) than that of complex (1). The IDA arm of chelate adopted fac-NO2 conformation in both complexes (1) and (2). Moreover, the photovoltaic properties as semiconducting nature for ligand and their corresponding Ni(II) complexes were estimated using Tauc’s equation, αhν = A(hν-Eg)r, where r = ½ for indirect and 2 for direct electronic transitions through UV–vis absorption data. The indirect and direct electronic transition bandgap (Eg) calculated for complex (1) is 2.0 and 3.07 eV respectively. Similarly, 2.55 and 4.39 eV respectively measured for complex (2).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


